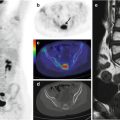
Fig. 12.1
(a, b) Whole-body123I-MIBG scan of a patient with stage IV neuroblastoma. The scan was performed before treatment was started
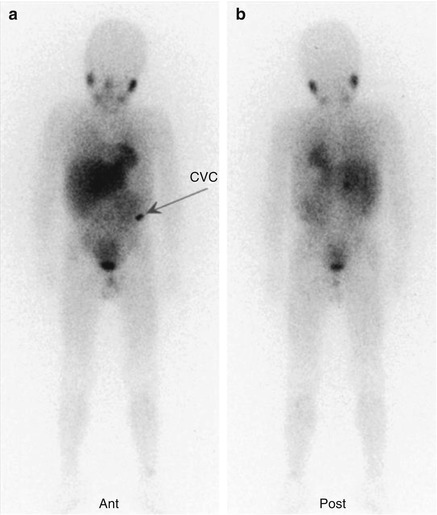
Fig. 12.2
The same patient as in Fig. 12.1. (a, b) This second whole-body scan was performed to monitor the response to induction therapy. The patient obtained a complete response (CR). Note the residual tracer stasis in the central venous catheter (CVC) reservoir (arrow in a)
12.2 PET Imaging in Neuroblastoma
12.2.1 Fluorodeoxyglucose
The principal PET tracer in oncology is undoubtedly 18F-FDG, and its role in NB has been accordingly investigated [21–27]. Its most frequent use has thus far been in patients with a negative or inconclusive 123I-MIBG scan (Fig. 12.3). In this setting, the superior performance of 18F-FDG–PET has proven, based on a sensitivity and specificity of 78 and 92 %, respectively, whereas for 123I-MIBG scintigraphy, the corresponding values are 50 and 75 % [24].
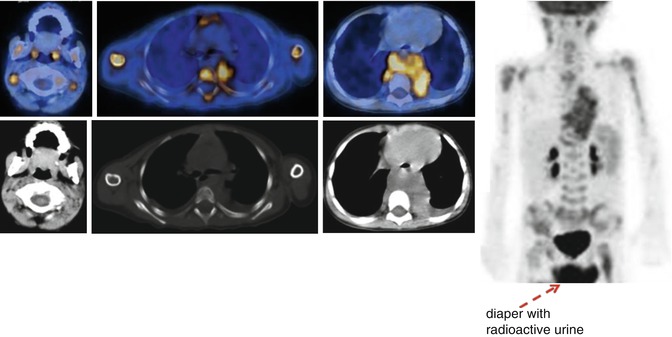

Fig. 12.3
18F-FDG–PET/CT staging in a patient with stage IV neuroblastoma. Note the large tumor in the chest, associated with massive bone marrow and multiple lymph node involvement
18F-FDG–PET has also been suggested as a complementary rather than a substitute exam for MIBG scintigraphy in NB staging and treatment monitoring [21–23]. Its diagnostic use to evaluate the response to therapy, especially in patients with high-risk, advanced stage disease, has been assessed [27]. The FDG-avidity of NB tumors increases with their aggressiveness and in those with an unfavorable histology, such that NB detection with 18F-FDG–PET is feasible and may even be superior to MIBG scintigraphy [24]. Further advantages of 18F-FDG–PET are its high resolution, short scanning period, and patient-friendliness. The principal limitations of 18F-FDG as a tracer for NB imaging are its overall low accuracy in the detection of disease in the bone and bone marrow, which are common sites of distant metastasis, the difficult visualization of disease occurring in the skull because of the intense physiological uptake of 18F-FDG in normal brain, and the reduced capability of 18F-FDG–PET to properly assess the response to therapy (Fig. 12.4) [25, 26].
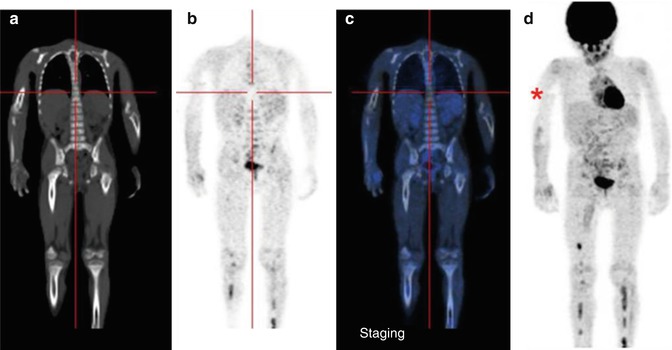
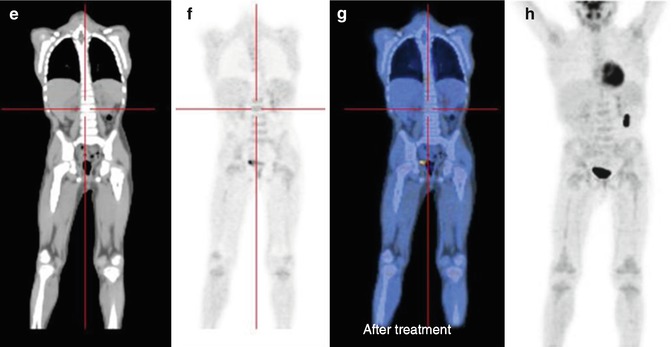


Fig. 12.4
Same patient as in Figs. 12.1, 12.2, and 12.5c, d. (a–d) 18F-FDG–PET/CT staging documents almost all disease sites, except the frontal lesion, which is masked by the intense physiological uptake of tracer in the brain. The red asterisk, seen on the MIP image, points to a technical artifact derived from movement during image acquisition. (e–h) Corresponding coronal and MIP views obtained with 18F-FDG–PET after the end of treatment. All previous disease sites now show normal uptake
Recently, new indications for this imaging method have been investigated, mainly based on the prognostic role of the FDG-avidity of tumors in patients with high-risk NB under consideration for 131I-MIBG therapy [27]. However, in that study, 123I-MIBG was shown to be superior in the detection of disease extent (Fig. 12.5). Both the SUVmax and the FDG-avidity of bone and bone marrow metastases were identified as adverse prognostic factors (Figs. 12.6, 12.7, 12.8, 12.9, 12.10, and 12.11).
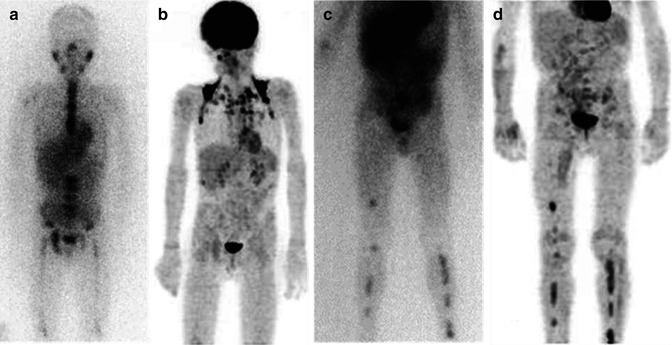
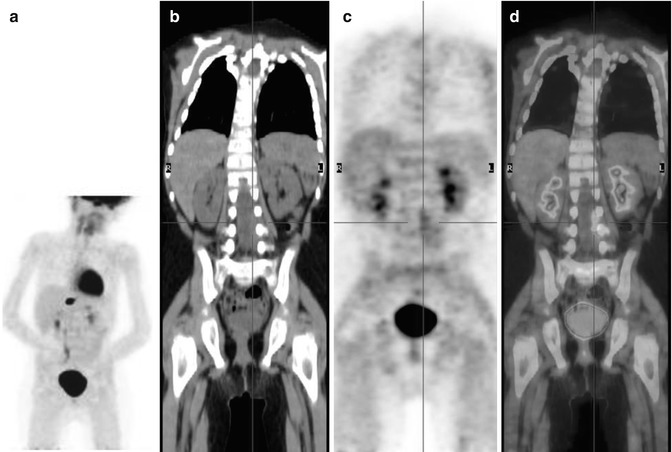
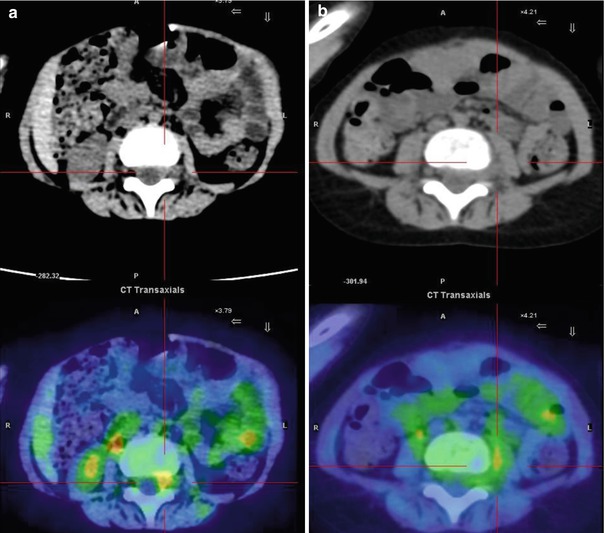
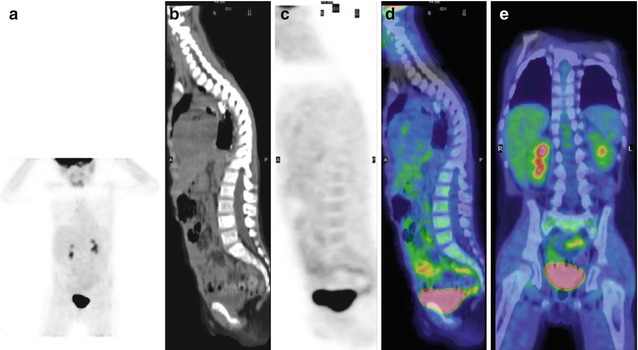
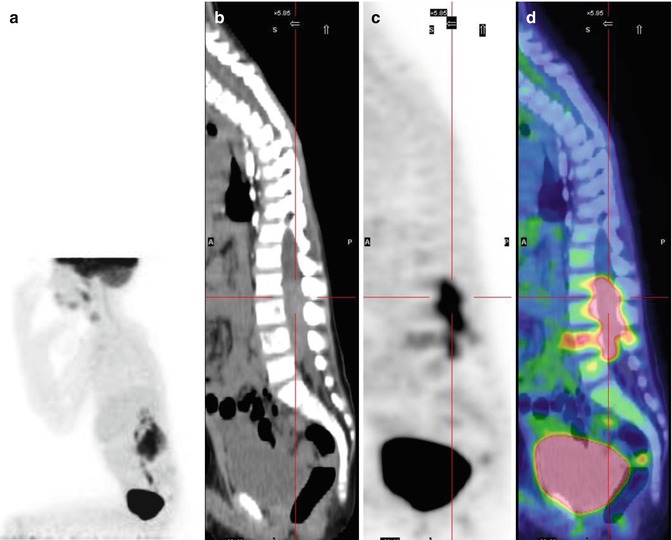
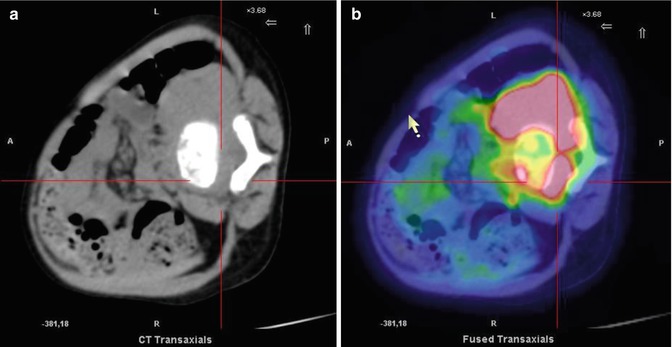
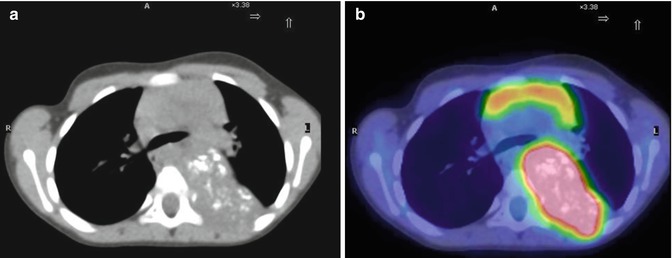

Fig. 12.5
Two different patterns of neuroblastoma detection, by means of 123I-MIBG (a, c) and 18F-FDG (c, d). Images (a, b) were obtained in the same patient. Note the extensive bone marrow involvement visible on the MIBG scan at the level of the spine, pelvic basin, and both femora, which is almost undetectable on the 18F-FDG–PET. Images (c, d) were obtained from another patient, during staging. Bone and bone marrow involvement of both legs is seen both on the 123I-MIBG scan and on 18F-FDG–PET

Fig. 12.6
A 5-year-old boy treated 3 years earlier for a thoracic neuroblastoma, stage IV, not amplified, 1p36 deleted. Following the development of pain in his right leg and difficulty walking, he underwent MRI, which showed the presence of epidural tissue in the L3–L5 vertebral canal. The bone marrow aspiration was negative, urinary catecholamines were normal, and 123I-MIBG scintigraphy was negative. (a) MIP; (b) coronal CT, (c) PET, (d) PET/CT fusion images show inhomogeneous 18F-FDG uptake in the left L3–L5 vertebral canal

Fig. 12.7
Same patient as in Fig. 12.6. Axial CT and PET/CT fusion images of the intra-canal (a) and extra-canal (b) lesion. The patient underwent chemotherapy followed by interleukin-2 and isotretinoin treatment

Fig. 12.8
Same patient as in Fig. 12.7, after chemotherapy. (a) MIP, sagittal (b) CT, (c) PET, (d) PET/CT, and (e) coronal PET/CT fusion images show the complete disappearance of any 18F-FDG uptake. Note the presence of movement artifacts on the MIP and coronal PET images, at the level of the neck–thorax

Fig. 12.9
Same patient as above. (a) MIP (position chosen to minimize the intense pain reported by the patient), sagittal (b) CT, (c) PET, and (d) PET/CT fusion images 7 months later show important disease relapse. The intensity and extent of 18F-FDG uptake are greater than in the first PET exam, suggesting the aggressiveness of the new tumor

Fig. 12.10
Same patient as above, now in the lateral position. (a) CT and (b) PET/CT fusion images show intense and extensive uptake at the level of the 4th lumbar vertebra. The lesion involved both the intra- and extra-canal areas. The child died 4 months later

Fig. 12.11
(a, b) A 4-year-old girl with neuroblastoma underwent a PET/CT study for staging. Note the presence of calcifications in the pathological mass, typical of this type of disease
12.2.2 18F-DOPA
In NB, the tumors typically produce biologically active hormones such as norepinephrine and several of its precursors, including dihydroxyphenylalanine (DOPA) and dopamine [28, 29]. 18F-dihydroxyphenylalanine (18F-DOPA), the radiolabeled formulation of dihydroxyphenylalanine, is a multivalent molecule widely used in the functional imaging of neuroendocrine tumors and the best PET alternative to 123I-MIBG because of its similar ability to follow catecholamine metabolism, which is increased in NB [30–34]. PET carried out with 18F-DOPA has a better diagnostic accuracy than either 123I-MIBG scintigraphy or conventional imaging modalities, such as CT and MRI, in the study of tumors excreting high levels of catecholamines (Fig. 12.12) [48–51], with a sensitivity vs. these latter methods of 90, 65, and 67 %, respectively [51].
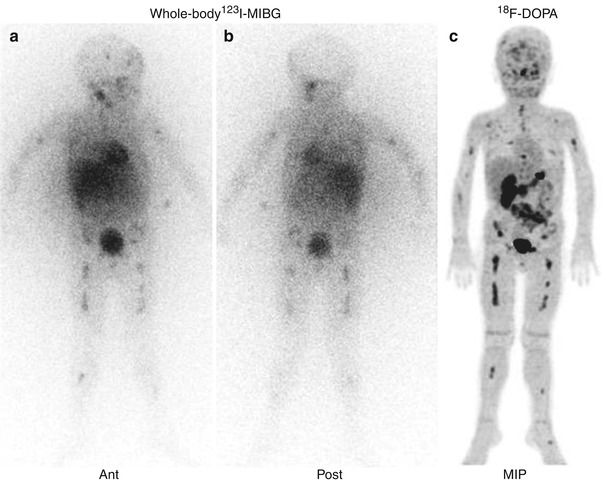

Fig. 12.12
Direct comparison of the whole-body 123I-MIBG scintigraphy (a, b) and 18F-DOPA–PET scan (c) of the same patient as in Fig. 12.3. Note the superimposable pathological distribution of the tracers visible on the two imaging modalities (Courtesy of Arnoldo Piccardo MD, Galliera Hospital, Genoa, Italy)
Consequently, several pilot studies have recently investigated the role of 18F-DOPA in NB patients. In a cohort of high-risk patients with primary/relapsed disease (n = 19), the 18F-DOPA distribution at NB sites was similar to that of 123I-MIBG [35], but the accuracy of 18F-DOPA–PET was higher than that of 123I-MIBG scintigraphy, especially for smaller lesions (<1.5 cm). This difference influenced patient management and treatment decisions in 32 % of the cases. In a direct comparison with morphological imaging (CT/MRI), 18F-DOPA–PET performed better (Fig. 12.13) [47].
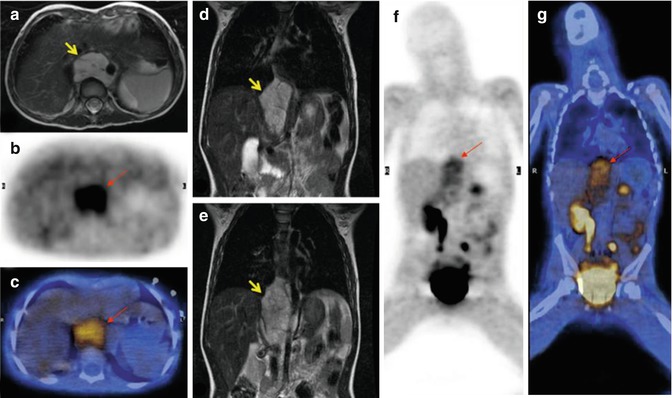

Fig. 12.13
18F-DOPA–PET/CT and fast spin-echo T2-weighted MRI scans of the same patient, who presented with a thoracic–abdominal neuroblastoma (arrows). (a, d, e) Axial and coronal MRI; (b, f) axial and coronal 18F-DOPA–PET images; (c, g) fused PET/CT images, axial and coronal views. Note also the important PET tracer retention in the excretory system, namely, the right renal pelvis, proximal ureter, and urinary bladder
While these findings are encouraging, they require further validation in larger, multicenter, prospective trials.
12.2.3 68Ga-DOTATOC
As with other neuroendocrine tumors, NB tumors overexpress somatostatin receptors (SSTRs), especially SSTR types 1 and 2 [36, 37]. This observation led to studies of 111In-pentetreotide scintigraphy or somatostatin receptor scintigraphy (SRS) in the assessment of NB [38, 39]; however, neither method was superior to 123I-MIBG. Instead, complementary roles, based on the ability of these methods to provide prognostic information, were recommended, as a positive SRS scan was shown to be a predictor of better outcome in NB patients [38, 39].
Recently, the use of PET tracers such as 68GA-DOTATOC to follow SSTRs in NB has been examined [40]. In a limited cohort comprising pheochromocytoma (n = 6) and NB (n = 5) patients, the accuracy of 123I-MIBG scintigraphy and 68GA-DOTATOC PET was investigated. According to a lesion-based analysis, the sensitivity of 68GA-DOTATOC and 123I-MIBG for NB was 97.2 and 90.7 %, respectively. Primary NB lesions were better definable in 68GA-DOTATOC PET imaging than in 123I-MIBG scintigraphy, suggesting the advantage of the former especially regarding the peptide receptor radionuclide therapy (PRRT) planning [41]. Undoubtedly, this indication for 68GA-DOTATOC must still be thoroughly assessed in children and the inclusion criteria precisely delineated before this imaging method replaces the already available and safe 131I-MIBG scintigraphy.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree