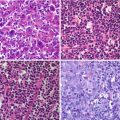
Fig. 11.1
(a, b) MIP (maximal intensity projection) images showing the physiological distribution of two different PET tracers, respectively, 18F-DOPA and 68Ga-DOTANOC. Along with the normal tissue activity marked by the arrows, 18F-DOPA PET reveals tracer stasis in the gallbladder, which is in part masked by the activity of the renal cortex
In oncology, 18F-DOPA is mostly employed in imaging tumors arising from neural crest cells and mimicking APUD (amine precursor uptake and decarboxylation) cells in their ability to accumulate and decarboxylate l-DOPA, as a precursor of dopamine [24]. Today, the main application of is in the study of NETs, both primitive and metastatic, including carcinoid (Fig. 11.2), GEP tract tumors, glomus tumors, medullary carcinoma of the thyroid, paraganglioma, and pheochromocytoma [25–29].
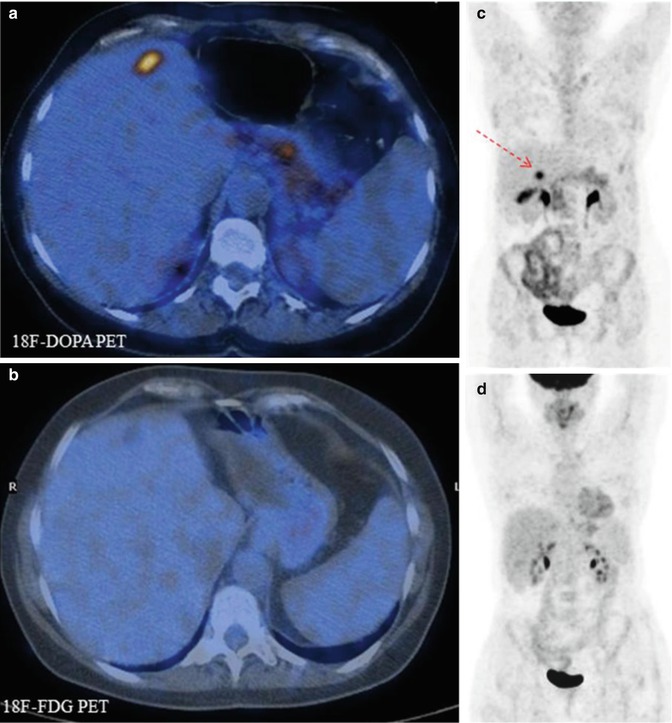

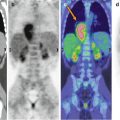
Fig. 11.2
Comparison of 18F-DOPA PET (a, b) and 18F-FDG–PET (c, d) scans in a patient with metastatic carcinoid. Note that the 18F-DOPA-avid lesion visible in the liver (c, arrow) does not show tracer uptake in the corresponding 18F-FDG–PET views (fused axial CT/PET and maximum intensity projections)
The diagnostic accuracy of 18F-DOPA PET in these types of neoplasia is very high, surpassing other methods of conventional and anatomic imaging such as CT and MRI, as well as functional scintigraphy with 123I-MIBG and 111In-octreoscan [30–32]
The group of neoplasms best evaluated with 18F-DOPA PET imaging are NETs that excrete large amounts of catecholamines, especially pheochromocytomas (Fig. 11.3), in which imaging sensitivity reaches 90 %, specificity 100 %, and accuracy 92 % [31, 32]. However, while these results are well documented in adults, the experience in children is very limited, although the superimposable behavior of these tumors in patients of all ages supports the applicability of the findings also in the pediatric population.
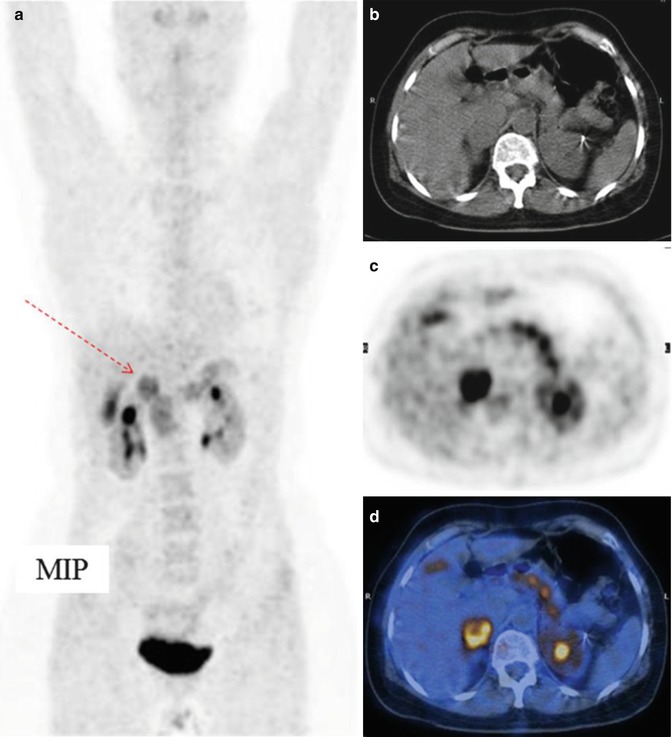

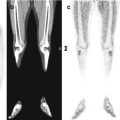
Fig. 11.3
Pheochromocytoma of the right adrenal gland (arrow), imaged by means of 18F-DOPA CT/PET. (a) Maximum intensity projection, (b) CT, (c) PET, and (d) CT/PET fusion image
11.4.2 68Ga-DOTA Peptides
The group of radiopharmaceuticals comprising 68Ga-DOTA peptides (-NOC, -TOC, -TATE) includes several octreotide analogues, all targeting somatostatin receptors with variable affinity (Fig. 11.1) [33, 34]. The rationale for using radiolabeled octreotide analogues in NET imaging is the finding that in >80 % of the cases, these tumors overexpress somatostatin receptors (SSTRs) [35, 36].
68Ga-DOTA peptides were first investigated for clinical purposes in 2001 [37], immediately followed by the development of several promising PET tracers [38, 39] for use in the diagnosis of primary NETs and in tumor staging (Figs. 11.4 and 11.5) [34, 40, 41]. Compared to other imaging modalities, such as 111In-octreotide or CT, the diagnostic accuracy of PET with 68Ga-DOTA peptides is outstanding, with a 97–100 % sensitivity and 96–100 % specificity [42–46].
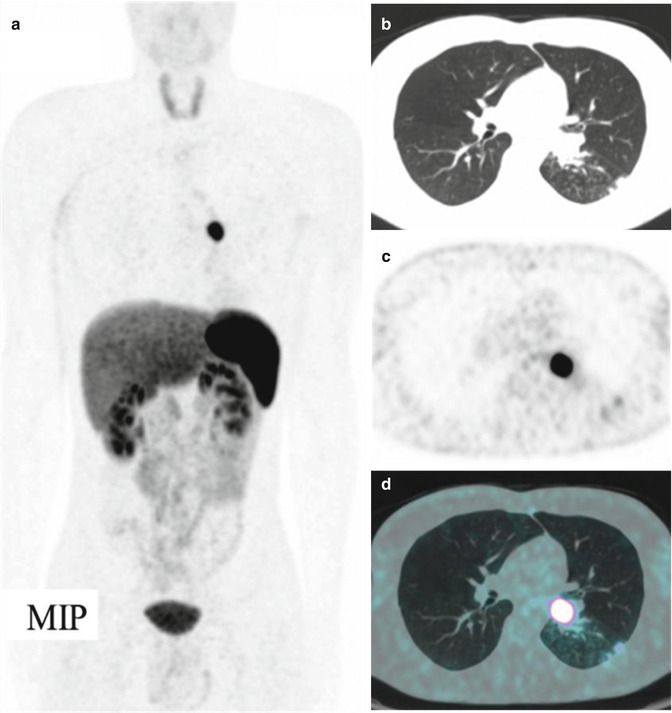
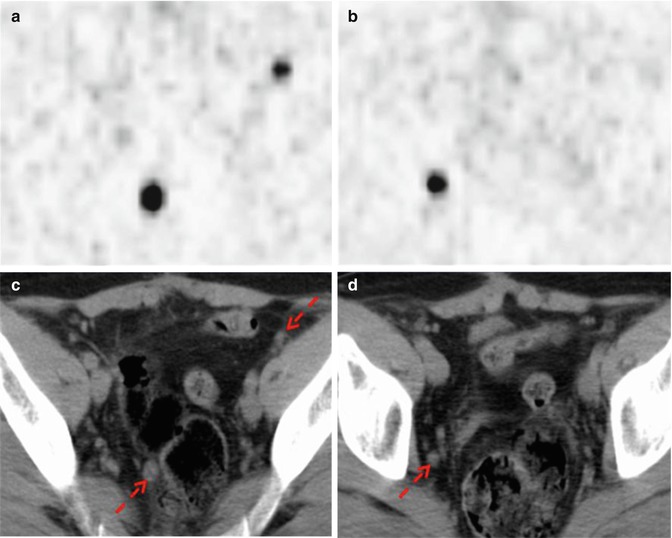

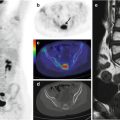
Fig. 11.4
A bronchial carcinoid at the level of the left lung hilus, imaged by means of 68Ga-DOTANOC PET/CT. (a) Maximum intensity projection, (b) CT, (c) PET, and (d) CT/PET fusion image

Fig. 11.5
68Ga-DOTANOC PET/CT shows multiple pelvic lymph node metastases deriving from a rectal carcinoid (red arrows). (a, b) Axial PET images of the pelvis; (c, d) corresponding low-dose CT
At staging or restaging, PET with 68Ga-DOTA peptides has a demonstrated capability to detect unknown metastases in up to 21.4 % of cases, often leading to significant changes in the management of these patients [42, 44, 47]. The 68Ga-DOTA-peptide uptake value (SUVmax) correlates with the clinical and pathological characteristics of NETs and is thus a significant prognostic factor in determining patient outcome [48].
Very recently, 68Ga-DOTA peptides were investigated in a pediatric population [49]. Despite the fact that the series was very small and restricted to pheochromocytoma (n = 6) and neuroblastoma (n = 5) patients, the rather promising results open the way to other applications of radiolabeled DOTA peptides in children, in particular peptide receptor radionuclide therapy (PRRT).
11.4.3 18F-Fluorodeoxyglucose
18F-fluorodeoxyglucose (18F-FDG) is the tracer of choice in the imaging of most malignant tumors, but its utility in NETs is limited because these tumors exhibit relatively low uptake of 18F-FDG as the vast majority of NETs are well differentiated [50, 51]. However, since tumor aggressiveness is positively associated with FDG-avidity, 18F-FDG–PET is advantageous in some types of NETs, i.e., those that are histologically dedifferentiated, or to confirm a poor prognosis [52].
11.4.4 Other PET Tracers
Although not yet used in the pediatric population, other PET tracers may be of clinical relevance when investigating NETs. For example, 11C-hydroxytryptophan (11C-HTP) [53] has been employed in the imaging of islet cell tumors, as has 18F-fluorodopamine (18F-FDA) [54] and 11C-hydroxyephedrine (11C-HED) [55], with very good diagnostic capability in pheochromocytoma and paraganglioma. However, one of the major limits of these radiopharmaceuticals is their relatively difficult synthesis and commercial availability, which limit their routine use in clinical practice.
References
1.
2.
3.
4.
5.
6.
7.
Spunt SL, Pratt CB, Rao BN et al (2000) Childhood carcinoid tumors: the St Jude Children’s Research Hospital experience. J Pediatr Surg 35:1282–1286PubMedCrossRef
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree