Neuroendocrine Tumor Imaging and Therapy
Erik S. Mittra
Hong Song
LEARNING OBJECTIVES
1. Understand the background of neuroendocrine tumors and physiologic basis for theranostics.
2. Describe the technique and interpretation of somatostatin receptor (SSTR) and metaiodobenzylguanidine (mIBG) imaging.
3. Understand the practical aspects of SSTR and mIBG therapy.
INTRODUCTION
Imaging of neuroendocrine tumors (NETs) has a long history in Nuclear Medicine (NM). Because NETs constitute a diverse group of tumor types, this field is broad. Earlier, however, there were only limited options for imaging and no food and drug administration (FDA)-approved therapies. Recently, there has been a resurgence of this area due to the FDA approval of one new imaging (in 2016) and two new therapeutic radiopharmaceuticals (both in 2018). This area is also an excellent example of the concept of theranostics, which has received much attention in NM over the last decade.
BACKGROUND
NETs include a wide range of tumors that arise from cells of the endocrine or nervous system and are well known for being heterogenous. They can arise from a variety of different organs, may be benign or malignant, are well-, moderately-, or poorly differentiated (e.g., their grade), have variable metastatic potential, and are either functional or nonfunctional (i.e., overproduce hormones causing symptoms or not) (1). Beyond disease progression, these symptoms can significantly reduce the quality of life (QoL) and are an important consideration for the need for therapy. NETs have low incidence, but a high prevalence given the relatively lower mortality associated with these tumors.
Regarding anatomic site or organ of origin, the most common site is the bowel (either foregut, midgut, or hindgut), followed by the pancreas, and by the lung. The first two are often grouped as gastro-entero-pancreatic (GEP) NETs and account for approximately two thirds of NETs. Those originating in the lungs can be further subdivided into bronchial, pulmonary carcinoid, small-cell lung cancer, and large-cell neuroendocrine carcinomas (NECs) of the lung. Other NETs include pheochromocytomas, paragangliomas, neuroblastoma, medullary thyroid cancer, and Merkel cell cancer.
The World Health Organization grading of NETs is based on cellular proliferation, either mitotic count (per 10 high-powered field) or the Ki-67 proliferation index (%). Low-grade (G1) tumors have a mitotic count of <2 or a Ki-67 of <3%. Intermediate-grade (G2) tumors have a mitotic count between 2 and 20 or a Ki-67 of 3% to 20%. And high-grade (G3) carcinomas have a mitotic count of >20 or a Ki-67 of >20%. It has been suggested that G3 be further subdivided into well- and poorly differentiated neoplasms to better reflect prognosis.
Nuclear imaging and therapy of NETs are based primarily on one of two features of these cell types. The first is somatostatin receptors (SSTRs) and the second is norepinephrine. The former is the basis of Indium-111 pentetreotide (OctreoScan) and Gallium-68 DOTATATE (NetSpot) imaging, and Lu-177 DOTATATE (Lutathera) therapy. The latter is the basis of I-123 metaiodobenzylguanidine (mIBG) imaging and I-131 mIBG or I-131 iobenguane (Azedra) therapy. Each of these is discussed in greater detail further in this chapter.
While not the focus of this chapter, it should also be noted that two other radiopharmaceuticals have a role in the imaging of NETs, which include 18F-fluorodeoxyglucose (FDG) and 18F-fluorodopa (FDOPA). As a glucose analog, 18F-FDG crosses the cell membrane via the glucose transporter and evaluates the glycolytic pathway of the cell. It is primarily useful for high-grade NECs which typically do not have high SSTR expression. Well-differentiated NECs (Ki-67 values in the range of 20%-50%) may need both 68Ga-DOTATATE and 18F-FDG imaging for full staging given the variable expression of SSTRs. FDOPA is a fluorinated form of levodopa and is taken up by NETs based on their ability to store biogenic amines and crosses the cell membrane via the large amino acid transporter. Studies have shown that 18F-FDOPA may have higher sensitivity than SSTR scintigraphy (2).
Somatostatin receptors
A primary commonality among many NETs (especially lung and GEP-NETs, more variable for other NETs) is their expression of the G protein-coupled SSTRs, of which there are five known subtypes in humans. Several peptides have been developed to bind with SSTRS, with variable affinity. Table 6.1 shows the relative binding affinity of a subset of these, focused on the imaging compounds.
Table 6.1 RELATIVE BINDING AFFINITY (IC50 IN NMOL/L) OF DIFFERENT SOMATOSTATIN RECEPTOR ANALOGS TO THE FIVE KNOWN SUBTYPES OF THE HUMAN SOMATOSTATIN RECEPTOR, HIGHLIGHTING THE DIFFERENCE BETWEEN PEPTIDES AS WELL AS THE EFFECT OF CHELATORS AND ISOTOPES (71) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
The human somatostatin molecule is a hormone used by neuroendocrine cells for neurotransmission and cell proliferation. It has two active forms, one composed of 28 amino acids and the other 14 amino acids. An abbreviated version of the human somatostatin molecule, composed of 8 amino acids, with a much longer plasma half-life, is octreotide (Sandostatin®). Octreotide was FDA-approved in 1988 for symptom control in patients with functional NETs (3). However, it is also known to have anti-proliferative effects and is practically used in that way for patients with low- or intermediate-grade, metastatic, inoperable NET (4). A slight variation of this peptide is lanreotide (Somatuline®), which is also used for the same indications.
To allow imaging and therapy of NETs, several variations of the octreotide peptide have been developed and labeled with gamma-, positron-, or beta-emitting isotopes for these various applications (5). The isotope connects to the peptide with one of two structural linkers, either diethylenetriaminepentaacetic acid (DTPA) or tetraazocyclodecanetetraacetic acid (DOTA). Aside from octreotide, the other peptide used is octreotate. These different radiopharmaceuticals (peptide-linker-isotope combinations) have different binding affinities to the various subtypes of SSTRs as well as dosimetry in normal organs (Table 1) (6,7,8,9). Ultimately, the images as well as lesional dosimetry are similar but not the same (4,7,10).
Somatostatin receptor imaging
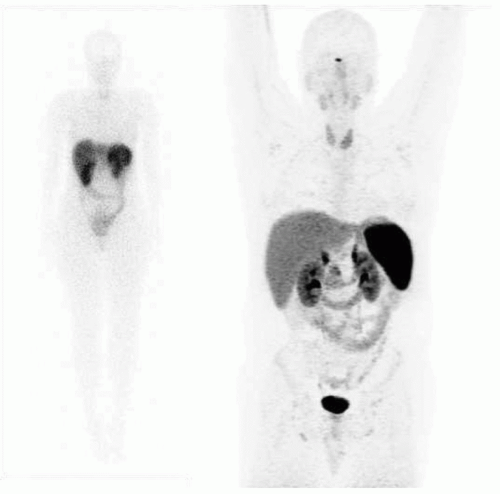
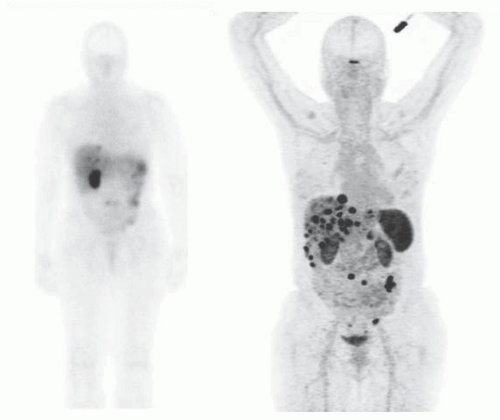
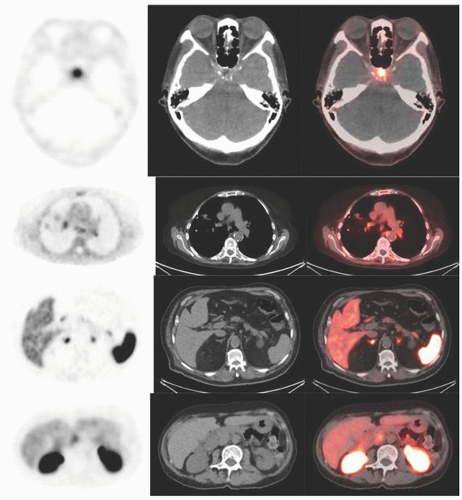
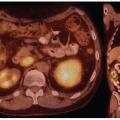
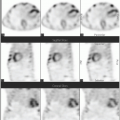
Historically, the first approach to both nuclear imaging and therapy of NETs was with Indium-111 DTPA-octreotide (or In111-pentetreotide; OctreoscanTM) (5). In-111 allows for gamma-imaging and this has been the mainstay of nuclear imaging of NETs since the 1980s. The imaging protocol is somewhat cumbersome as the radiopharmaceutical injection is followed 24 and (often) 48 h afterward with whole-body planar imaging with or without SPECT/CT. Even further delayed imaging is sometimes necessary and the patient is requested to have regular bowel movements during this time (with the use of over-the-counter laxatives if needed) to increase the specificity of the scan. The next generation of imaging agents used the positron-emitting isotope Gallium-68 (68Ga) bound to either octreotide or octreotate using the DOTA-linker (6). Three peptides based on this concept include DOTA-TATE, DOTA-TOC, and DOTA-NOC. In June 2016, 68Ga-DOTATATE (NetSpot, Advanced Accelerator Applications [AAA]) was FDA approved, thereby providing a PET-imaging alternative to the long-used 111In-pentetreotide (Figs. 6.1,6.2,6.3). Aside from the improved resolution of positron emission tomography (PET) over planar as well as SPECT gamma camera imaging, the imaging protocol is also much more favorable as the whole-body scan is done at 60 min post injection. In fact, in all key aspects (resolution, imaging time, radiation dose, sensitivity, and resultant change in clinical management), 68Ga-DOTATATE is superior to 111In-pentetreotide and should, therefore, be used whenever possible. There are still some areas that are struggling with the availability of Ga68-DOTATATE locally, or insurance denials, but these issues will become less in the future.
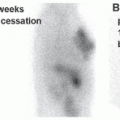
There is some controversy about the proper preparation for these scans, with regard to stopping octreotide or lanreotide therapy. Since those medications will compete with the same SSTRs for imaging, they can theoretically cause a reduction in uptake and, therefore, decrease the sensitivity of the scan. Results are somewhat mixed, but the general guidance is to stop short-acting medications for 24 h before imaging, and long-acting medications for 1 month before imaging, if possible.
Somatostatin receptor therapy
Therapy is also possible with In-111 from the auger electrons that are produced by the photons, which results in DNA damage, and this was the first therapeutic approach tried. However, efficacy was low. As such, the next generation of therapeutic radionuclides used were the beta-emitting isotopes Yttrium-90 (Y-90) and Lutetium-177 (Lu-177) bound to the same linker and peptide combinations described earlier for imaging. Both of these therapeutic isotopes are still in use today (more so in Europe than in the United States) although Lu-177 is the only one that has both FDA and European medicines agency (EMA) approval and is commercially available.
While both Y-90 and Lu-177 are primarily beta-emitting radioisotopes, there are some differences between them. Y-90 has a half-life of 2.7 days, energy of 935 keV, a path length of 12 mm in soft tissue, and no primary gamma emission. Lu-177 has a half-life of 6.7 days, energy of 133 keV, path length of 2 mm in soft tissue, and additional 113 keV (6.6%) and 208 keV (11%) gamma emission. Primarily, it is theorized that the higher energy (and the resulting longer path length) of Y-90 will provide greater efficacy for larger tumors (11). This has been investigated in several clinical trials (12). Unfortunately, the longer path length can also result in greater toxicity to surrounding normal tissue such as bone marrow or the kidneys, although this is still limited (11). As such, Lu-177 was chosen for commercialization given similar efficacy but lower toxicity, as compared to Y-90.
The use of a radionuclide attached to a peptide for the purpose of therapy is generally termed peptide receptor radionuclide therapy (PRRT). It is not specific to NETs, but NET therapy using a somatostatin analog attached to Lu-177 is currently the most advanced and only FDA-approved example of PRRT. Furthermore, this combination of imaging and therapeutic isotopes utilizing the same target is a good example of theranostics, which is a portmanteau of the words therapy and diagnostics, and is a powerful concept in the field of NM.
CLINICAL TRIALS OF PEPTIDE RECEPTOR RADIONUCLIDE THERAPY
Phase I or II experience
Because of regulatory and funding differences between the United States and Europe, much of the initial work on PRRT for NETs has been done in Europe, primarily in the Netherlands and Germany, as well as a few other places in the world. The first patient treated with 177Lu-DOTATATE was in 1997, and since then, various sites in Europe have gained considerable experience with this therapy. While valuable, the majority of the studies have been smaller Phase I and II trials.
Even very early prospective studies on 177Lu-DOTATATE with only 35-50 patients showed partial response rates of 10% to 25%, as well as improvements in QoL measures in those with metastatic NET who were refractory to traditional therapies (13,14). Follow-up studies in much larger (500-600 patients, but single institution) cohorts continued to show favorable outcomes with 30% objective response rates, 40-month progression-free survival
(PFS), and low G3 and G4 toxicities (15). Recent publications from these groups focus on long-term tolerability and outcomes (again in larger groups of 400-800 patients), showing favorable toxicity profiles regarding the kidneys and bone marrow (with greater renal toxicity in those treated with Y-90 vs. Lu-177) and low levels of significant toxicity otherwise, with the suggestion for individual predilections toward radiation (16). The worst outcomes were very small percentages of patients developing acute leukemia (<1%) or myelodysplastic syndrome (<2%), in the context of PFS of 29 months and overall survival (OS) of 63 months (17,18).
(PFS), and low G3 and G4 toxicities (15). Recent publications from these groups focus on long-term tolerability and outcomes (again in larger groups of 400-800 patients), showing favorable toxicity profiles regarding the kidneys and bone marrow (with greater renal toxicity in those treated with Y-90 vs. Lu-177) and low levels of significant toxicity otherwise, with the suggestion for individual predilections toward radiation (16). The worst outcomes were very small percentages of patients developing acute leukemia (<1%) or myelodysplastic syndrome (<2%), in the context of PFS of 29 months and overall survival (OS) of 63 months (17,18).
Neuroendocrine tumors therapy trial
Based on many favorable Phase I/II results, AAA funded the registry trial (neuroendocrine tumors therapy trial [NETTER-1]) for 177Lu-DOTATATE, with the brand name Lutathera. This was the first Phase III multicenter, randomized, controlled, trial of Lutathera (19). It was simultaneously launched in both Europe and the United States, with a goal of both EMA and FDA approval, respectively.
The study design for the NETTER-1 trial is as follows. Eligibility was for adult participants with biopsy-proven, low-grade (Ki-67 <20%), metastatic or locally advanced, midgut NET, which was inoperable and radiographically progressing on standard dose (30-40 mg) octreotide therapy. Confirmation of SSTR expression was based on planar OctreoScan imaging since 68Ga-DOTATATE PET was not available in most centers at this time.
Participants randomized to the experimental arm received four doses of 200 mCi (7.4 GBq) of Lutathera once every 2 months. Importantly, long-acting octreotide was held for 1 month before each dose of Lutathera, but could be given 4 to 24 h after completion of the Lutathera infusion. Participants randomized to the control arm did not receive placebo. Rather, they received high-dose (60 mg) octreotide, which has a potential therapeutic benefit as well. The primary endpoint of the trial was PFS, with multiple secondary endpoints including OS, objective response rate, toxicity, and QoL measures.
A total of 229 patients were enrolled in the study; 116 on the experimental arm, and 113 on the control arm. Overall, the results were very favorable. The primary endpoint was reached with a strong difference between the two arms. Subjects receiving Lutathera had a 79% reduction in risk of progression (hazard ratio of 0.21, p < 0.001), with an estimated PFS of 40 months, compared to 8.4 months on high-dose octreotide therapy.
At the time of the initial NETTER-1 publication (as well as this study), the OS data were not finalized, as the 5-year follow-up phase is still ongoing. But the interim analysis showed a 60% lower risk of death with Lutathera (hazard ratio of 0.40, p < 0.004). The objective response rate was also significantly different between the two arms, with 1 complete response and 17 partial responses with Lutathera, and no complete response and 3 partial responses with high-dose octreotide. Lastly, the QoL measures showed improvements overall, as well as specifically for diarrhea, flushing, and abdominal pain.
177Lu-DOTATATE THERAPY
Patient selection
The FDA approval of Lutathera is for the treatment of SSTR-positive GEP-NETs, including foregut, midgut, and hindgut NETs in adults (20). It should be noted again that this therapy can also be used for other NETs such as those arising from the lung, as well as pheochromocytomas, paragangliomas, medullary thyroid cancer, and Merkel cell carcinomas, but the data are much more limited, and so is not the focus of this chapter. However, these applications are indicated in the joint International Atomic Energy Agency (IAEA), European Association of Nuclear Medicine (EANM); Society of Nuclear Medicine and Molecular Imaging (SNMMI) guidelines for this therapy (21). The primary considerations for patient selection for this therapy are the tumor grade, SSTR density based on nuclear imaging, operability, distribution of disease, progression, and laboratory values.
Tumor grade and SSTR density based on imaging are linked concepts. The therapy is most effective in patients who have high expression of SSTRs on their tumor cells (22). This is typically true for well-differentiated tumors (23,24), which is why the NETTER-1 trial required patients to have either low- or intermediate-grade tumors (Ki-67 <20%). Poorly differentiated or high-grade carcinomas (Ki-67 >20%) are more variable in their SSTR expression. Those in the Ki-67 range of 20% to 50% may have high-enough SSTR expression to warrant PRRT, while those above 50% generally do not. The assessment of SSTR expression is based on 111In-pentetreotide (OctreoScan) gamma-camera and SPECT imaging or, preferably, 68Ga-DOTATATE PET imaging. The majority of the patient’s lesions should have uptake greater than the liver background to be eligible for the therapy. There are ongoing studies on specific standardized uptake value (SUV) values or cutoffs to use for therapy and how it relates to outcomes (25). It is also worthwhile to note that because of those issues, 18F-FDG PET may also have a role in the determination of proper patient selection and response assessment for these patients (26), especially those with high-grade tumors.
Since partial or complete surgical removal of tumors is always preferred when possible, PRRT is reserved for patients with locally aggressive and inoperable diseases. Furthermore, the disease is typically metastatic to multiple sites making other approaches such as liver-directed therapy, or external beam radiation, less appealing. Disseminated metastases within the liver are another example where PRRT should likely be favored over liver-directed therapies alone. Lastly, the disease needs to be progressing on standard-dose SSTR therapy with either Octreotide or Lanreotide. In the clinical trials, progression was typically confirmed radiographically (with either CT or MR) using response evaluation criteria in solid tumors (RECIST) criteria. Another area of consideration here is the role of OctreoScan or 68Ga-DOTATATE PET to show progression (e.g., when the disease is radiographically stable or only slightly enlarging, but the SUVs on PET imaging have increased significantly). This is presently an area of ongoing research.
Patients should meet certain laboratory values to ensure that potential transient collateral damage to the bone marrow and the kidneys will not be an issue. The laboratory cutoffs from the NETTER-1 and early access program (EAP) trials were as follows, and it would be reasonable to continue to check these same values for clinical patients at screening, and during the therapy. Patients should have a serum creatinine <1.7 mg/dL (or a creatinine clearance >50 mL/min calculated by the Cockroft-Gault method), hemoglobin >8 g/dL, white blood cell count >2000/mm3, platelets >75,000/mm3, total bilirubin less than three times the upper limits of normal, and a serum albumin >3 g/dL, unless the prothrombin time is within the normal range.
Sequencing therapy
Even if a patient is eligible for PRRT based on the previously provided guidelines, it may not be the best choice relative to
other therapeutic options such as surgery, mTOR inhibitors, chemotherapy, external beam radiation, or a variety of liver-directed therapies (bland embolization, chemoembolization, or radioembolization) for those with liver dominant disease. This area is an active area of discussion and research, and the consideration is not only what will have great therapeutic benefit, but also which will have less toxicity now and in the future (23). Another possibility is combination therapies, which may have a greater benefit than the sum of their parts, for instance, by using radiosensitizing chemotherapies in conjunction with radioembolization or PRRT (27).
other therapeutic options such as surgery, mTOR inhibitors, chemotherapy, external beam radiation, or a variety of liver-directed therapies (bland embolization, chemoembolization, or radioembolization) for those with liver dominant disease. This area is an active area of discussion and research, and the consideration is not only what will have great therapeutic benefit, but also which will have less toxicity now and in the future (23). Another possibility is combination therapies, which may have a greater benefit than the sum of their parts, for instance, by using radiosensitizing chemotherapies in conjunction with radioembolization or PRRT (27).
The European Neuroendocrine Tumor Society guidelines have long recommended PRRT as a second-line therapy after progression on SSTR therapy, and this is a very reasonable approach to consider, with the caveats provided earlier. Ultimately, the therapy sequencing decision should ideally be done in the context of a multidisciplinary conference with experts from all the disciplines (oncology, surgery, radiation oncology, NM, radiology, and pathology) represented (1). Since in many places it is difficult to have so many NET experts, progressive NET patients should be referred to a NET Center of Excellence (aka NET Advanced Care Center), at least once in their care.
Performing the therapy
As compared to other radioisotope therapies such as I-131, Ra-223 dichloride (Xofigo), and I-131 ibritumomab tiuxetan (Zevalin), PRRT is significantly more involved. Having said that, once the therapy program has been set up at an institution, it is relatively straightforward and requires only a reasonable amount of input.
As mentioned earlier for imaging, but even more important for therapy, is to stop octreotide/lanreotide therapy for the appropriate amount of time (24 h for short-acting and 1 month for long-acting formulations) before treatment. The Lutathera is shipped from a radiopharmacy (either in Italy or in New Jersey) to the clinical site either the day before or on the day of the therapy and arrives as a clear liquid in a glass vial. The 177Lu-DOTATATE is then administered over 30 minutes, with another 10 to 20 minutes for infusion of saline to minimize the residual. There are two main recommended methods for administration. The first is referred to as the gravity method and requires the use of two needles inserted into the vial. The instillation of saline (either running via gravity or through a pump) through one needle increases the pressure within the vial and pushes the Lutathera out the other needle, which is attached to the patient. The second method is to manually draw out the contents of the vial into the syringe and then using a shielded, automated syringe-pump to administer the therapy to the patient.
The 177Lu-DOTATATE needs to be given in conjunction with an amino acid (AA) formulation, which is given intravenously through either the same or a second IV line. AAs reduce the residence time of 177Lu-DOTATATE in the kidneys thereby reducing radiation toxicity. Recently, there are various AA formulations available with different amounts of AAs, as well as different osmolalities. The only two necessary AAs are lysine and arginine. More pure formulations of just these two AAs, together with a lower osmolality solution, significantly reduce the nausea associated with their infusion. Depending upon which AAs are used (and their emetogenic potential) and the patient’s sensitivity, a variable amount of anti-nausea pre medication needs to be given before starting the AAs. Additional PRN anti-nausea medication may also have to be given during the infusion.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree