7 Neuroendovascular Procedures for Skull Base Neoplasia Neuroendovascular procedures in the setting of skull base neoplasia are typically performed to achieve preoperative tumor embolization. They are also occasionally performed to treat emergent complications arising from tumor pathology or treatment. The primary skull base tumors that most commonly benefit most from angioembolization are meningioma, juvenile angiofibroma, and paraganglioma.1 However, other hypervascular tumors may also benefit (hemangiopericytoma, chordoma, plasmacytoma, olfactory neuroblastoma, and metastasis especially renal and thyroid carcinoma). The goal of tumor embolization is to reduce the tumor vascularity.2 A variety of embolic agents have been used. The most common agents include temporary or semi-permanent agents like polyvinyl alcohol (PVA) and gelfoam and permanent liquid embolic agents such as n-butyl-cyanoacrylate (NBCA), ethylene vinyl alcohol copolymer (Onyx), and, in certain cases, coils.3 Following preoperative embolization, it is recommended that surgery be performed within 72 hours of the procedure in order to maximize the effect before collaterals and tumor neoangiogenesis can reperfuse the tumor. The goal of preoperative embolization is to increase the safety of subsequent surgical procedures by limiting intraoperative hemorrhage, reduce the need for blood transfusion, increase visibility in the surgical field, and shorten hospitalization length. Embolization sessions typically begin with angiography to document the tumor supply and assess vascular collaterals. Thereafter, dedicated angiography of the vessel to be embolized is performed to exclude the presence of dangerous anastomoses. Upon discovery of such an anastomosis, the channel can be closed with coils and embolization may then proceed safely, or the pedicle can be abandoned. Once a safe approach has been established, the embolic material of choice is injected under fluoroscopic control. In situations where there is encasement of large arteries like the internal carotid artery (ICA) or vertebral, where inadvertent or deliberate sacrifice may be anticipated, preoperative balloon test occlusions may be performed. Neuroendovascular embolization is also of utility in the setting of spontaneous hemorrhage from skull base neoplasia (e.g., epistaxis) or posttherapeutic vascular injuries such as carotid blowout/large artery laceration. There is a high morbidity associated with open surgical exploration and vessel coagulation/ligation in these situations. Hence, where feasible, endovascular techniques are now the preferred modality to address such problems. This chapter will discuss the clinical and radiological issues as they pertain to preoperative embolization of the commonly treated primary skull base tumors (meningioma, juvenile angiofibroma, paraganglioma) and then detail the general neurointerventional procedural protocol which can be applied to other tumors. Meningiomas are extra-axial tumors that commonly occur as derivatives from arachnoid cell degeneration and dural fibroblasts. Their origin from arachnoid cells leads to their common occurrence intradurally4; however, they can also occur at the skull base, or rarely in the neck along the carotid sheath. Meningiomas are generally benign lesions, frequently identified incidentally on autopsy, and possess small likelihood of becoming malignant over time. As the most common benign intracranial neoplasms, they represent about 15% of central nervous system (CNS) neoplasms, with a slight female predominance (2:1), partially attributable to the presence of estrogen and/or progesterone receptors.5 There is an established genetic correlation with neurofibromatosis (NF) type 2 in which case they may be associated with bilateral acoustic neuromas and schwannomas in an autosomal-dominant pattern of inheritance. They can also be familial and independent of NF-2. Meningiomas generally present in middle-aged patients ranging from ages 25 to 65 years, peaking in incidence at age 45 years. Multiple meningiomas occur far less frequently, in 1 to 2% of cases, predominantly affecting the central form of NF. Meningiomas have been seen up to 25 years after cranial radiation therapy, and these present more aggressively and in greater numbers with higher recurrence. These typically indolent tumors do not usually invade local structures in the brain, but are able to cause compressive symptoms including vision changes, headaches, seizures, and hormonal deregulation if the pituitary is involved. They may encase and narrow vessels. Malignant meningioma is a rare but aggressive variant, with the capability of invading the brain and producing distant metastasis. Meningiomatosis is a subtype that is multifocal with an atypically early and aggressive presentation. Hemangiopericytomas are another variant with angiomatous characteristics, though current thinking is that they may belong in the solitary fibrous tumor spectrum of disease. En plaque meningiomas have a high incidence in females and are characteristically osteogenic with minimal compressive symptoms. Meningiomas can occur in many locations including the convexity dura, dural sinus, anterior cranial fossa, parasellar, sphenoid wing, cavernous sinus, and posterior fossa. Intraventricular meningiomas are exceedingly rare, and when present are often located in the lateral ventricles. Orbital meningiomas usually involve the optic nerve sheath and can result in decreasing visual acuity, optic atrophy/paresis, and exophthalmos. Meningiomas of the central skull base and cavernous sinus deserve special mention as they can be difficult to manage due to the encasement of critical structures such as cranial nerves III, IV, V, VI, the ICA, the sellar contents, and the optic apparatus. Tumors in this location are often fed not only by external carotid artery (ECA) branches, but also by direct meningeal tributary arteries from the ICA, including the inferolateral trunk and the meningohypophyseal trunk. While the ECA supply can be embolized, embolization of ICA feeders is less safe because direct selection of these small dural feeders is often not possible, and there is risk of reflux of embolic material into the ICA. Occasionally, a balloon may be inflated in the cavernous sinus distal to these branches to redirect particles into the tumor, but the risk-to-benefit ratio of such maneuvers is questionable. Ischemic complications can occur if there is unintentional migration of embolic material into branches supplying the brain parenchyma or cranial nerves, resulting in neurological deficits. Ischemic necrosis from occlusion of arteries supplying the skin of the face or scalp can also occur. It should be noted that ischemia to cranial nerves and scalp necrosis is probably more common with liquid embolics such as onyx or NBCA, which have the ability to penetrate deeper into the microvasculature, as compared to particles. A preoperative occlusion test may be performed if there is the anticipated possibility of occlusion or laceration of the ICA during surgery. In patients who pass the test, intentional removal en bloc with either preoperative endovascular or intraoperative surgical sacrifice of the ICA may be preferable to trying to dissect the tumor off the artery. If the preoperative test occlusion fails, a subtotal resection with a cuff of tumor being intentionally left along with the vessel can be done. Alternatively, a vessel sacrifice and bypass type procedure can be contemplated (albeit with higher morbidity). Postembolization tumor swelling and intratumoral hemorrhage are also known possible complications, due to excessive intralesional ischemic necrosis.6 The rate of complication from meningioma devascularization has been reported between 2.5 and 6.4%.7 The complications include excessive ischemic necrosis leading to postoperative hemorrhage, and migratory embolization commonly through arterial anastomosis between dural and nonparenchymal structures, leading to neuroparenchymal or extracranial ischemia.8 Hemorrhagic complications are usually due to venous obstruction from embolic material or edema, or rupture of frail feeding vessels. An outline of the expected common feeder vessels for various anatomic locations of meningioma is noted in The benefits of preoperative embolization of meningiomas are heavily contingent on their anatomic location, which in turn influences their vascular supply. The goal of embolization is a reduction in perioperative blood loss. The majority of convexity meningiomas do not require embolization due to their superficial and thereby readily, surgically accessible blood supply (e.g., superficial temporal, occipital, middle meningeal, posterior meningeal arteries).9 The goals with preoperative embolization of meningiomas overlap with skull base neoplasms in general, including but not limited to reduction of tumor vasculature, minimizing blood loss, improved surgical field of view, and attenuation of tumor dimensions. PVA particles are usually favored for initial use, starting with smaller 50 to 150 microparticles if there is a low risk of collateral occlusion and necrosis and increasing in size if there is high flow shunting into veins. The embolization of vessels that are inaccessible surgically are especially useful when possible ( Table 7.1 Meningioma vascular supply by anatomical region
7.1 Introduction
7.2 Meningioma
 Table 7.1.
Table 7.1.
 Fig. 7.1).
Fig. 7.1).
Meningioma location | Predicted main vascular supply |
Convexity | Middle meningeal artery (MMA) |
Parasagittal | MMA |
Olfactory groove | Internal carotid artery (ICA) dural branches, ophthalmic artery, and ethmoidal branches |
Tentorium and clivus | Cavernous ICA, MMA, tentorial artery, artery of Davidoff/Schecter |
Posteromedial posterior fossa | Meningeal branches of occipital artery, ascending pharyngeal artery |
Lateral posterior fossa | MMA, falcotentorial branches |
Falcine | Terminal MMA branches, anterior falx artery branches |
Intraventricular | Choroidal arteries |
Sphenoid wing and clinoid | MMA |
A particular concern when managing petrous branches of the middle meningeal artery (MMA) arises due to the anastomoses with the vasa nervosum of the facial nerve (CN VII). Similar concerns arise when embolization is performed in the neuromeningeal trunk of the ascending pharyngeal artery, which supplies the vasa nervosum of lower cranial nerves. It is important to avoid smaller microparticles and liquid embolic agents in these instances. Particles greater than 150 µm are suggested in such pedicles.
Large-scale studies involving embolization of meningiomas reveal complication rates in the range of less than 2%.8 Brain ischemia (nontarget embolization) is the most feared complication and is rare. The degree of devascularization and therefore surgical benefit endowed by embolization may be dependent on tumor size. Previous studies have shown skull base meningiomas less than 6 cm that underwent embolization had significantly reduced blood loss, while those less than 6 cm showed less significant differences. This may be attributable to the abundant collateral blood supply with larger tumors.10 However, as skull base meningiomas are often supplied by branches of the ICA and vertebrobasilar system, inability to safely embolize these pedicles may have hindered successful devascularization, and account for the less effective devascularization occurring with larger meningiomas.
In more aggressive or advanced cases where tumor resection is not feasible or would inflict an unjustifiable degree of complications, an aggressive embolization strategy may be appropriate. If a safe vascular pedicle is readily accessible, significant tumor necrosis can be induced by embolization with alcohol or NBCA. Sometimes this can even eliminate the necessity of surgical intervention; however, there is very limited data on the safety or effectiveness of this approach.
Radiofrequency and cryoablative techniques have also shown promise in patients who are either poor surgical candidates or fail surgical attempt at cure and subsequent chemoradiotherapy. Other types of minimally invasive ablative therapies have been entertained such as MR-guided laser ablation, laser interstitial thermal therapy,11 and chemoembolization. Some of these techniques have been applied to other tumor types as well.12
7.3 Juvenile Nasopharyngeal Angiofibroma
Juvenile nasopharyngeal angiofibromas (JNAs) are rare benign tumors of the head and neck.13 They most commonly affect adolescent males aged 7 to 29 years, peaking in incidence at ages 14 to 17 years.14 Current literature does not indicate an ethnic or geographic predisposition, and cases involving females or older male patients should include a broader differential diagnosis.
JNAs are comprised of irregular blood vessels with distorted architecture set in a fibrous stroma. JNAs are benign but can exhibit locally aggressive behavior. Tumors that have not undergone radiation show exceedingly rare transition into malignancy. Their source of origin remains controversial, but these tumors mostly originate from the posterior nasal cavity, in close proximity to the sphenopalatine foramen. Rather than direct invasion of adjacent bone, gradual remodeling occurs. Typical extension is laterally through the sphenopalatine fossa, pterygomaxillary fossa, and retroantral infratemporal region resulting in the characteristic “antral bowing.” As the tumor grows, local invasion into the oropharynx, nasal cavity, maxillary or ethmoid sinuses, and orbit may occur. Similarly, superior extension into the sphenoid sinus, sella, cavernous sinus, and middle cranial fossa are possible. Orbital extension is typically extraconal and intracranial extension is typically extradural.
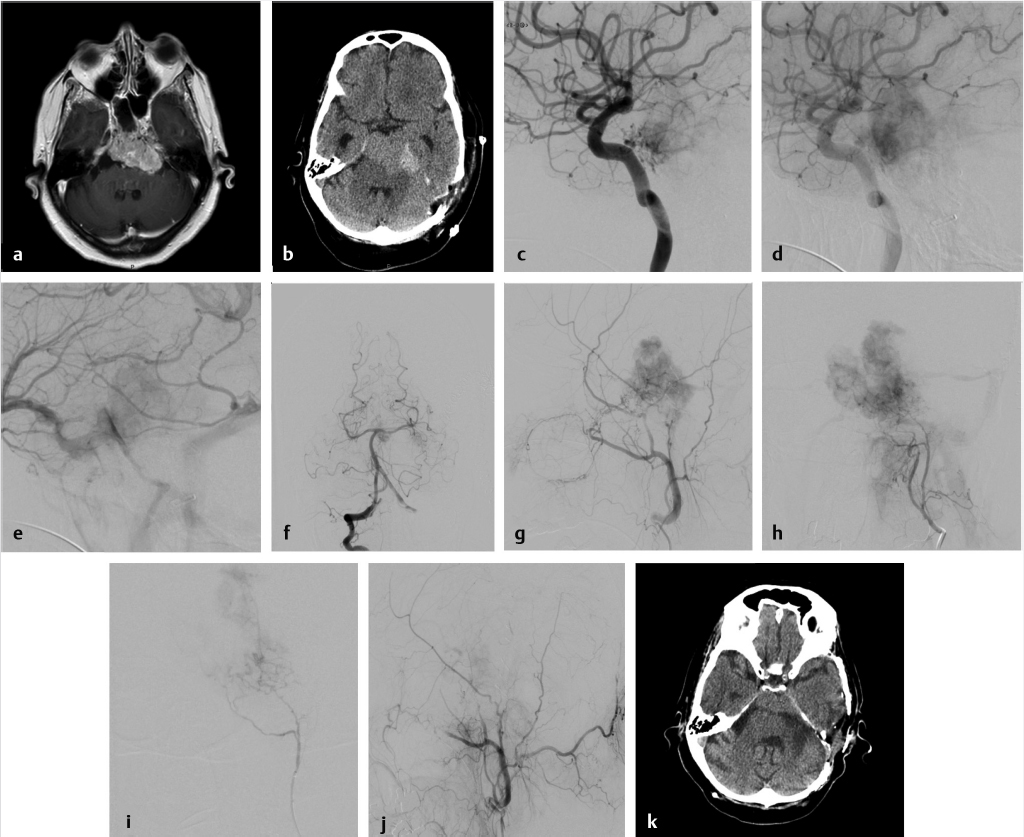
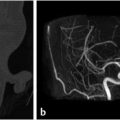
Fig. 7.1 Preoperative embolization of skull base meningioma. (a) Axial T1 postgadolinium shows a large petroclival meningioma involving the left cavernous sinus and Meckel’s cave presenting with symptomatic brainstem compression. (b) Initial staged resection was performed and the tumor was found to be highly vascular. Postoperative noncontrast computed tomography (CT) shows hemorrhage in the surgical bed with similar tumor bulk. The patient was referred for embolization in advance of further resection. (c–e) Early and late arterial and venous phase of a left internal carotid artery (ICA) angiogram shows supply to the tumor from dural (meningohypophyseal and clival) branches of the ICA. Note early arterial blush with late persistence into the venous phase, typical for meningioma (the “in-law sign”… comes early, stays late!). These branches are too small for superselective catheterization. Embolization with particles could be performed with a balloon inflated distally in the cavernous ICA followed by robust aspiration prior to balloon deflation; however, this is not without risk of inadvertent embolization. (f) Right vertebral artery injection shows minimal supply to tumor from tiny branches parasitized from the basilar and posterior cerebral artery (PCA; not amenable to embolization). (g) Left external carotid artery (ECA) injection shows robust supply to the tumor from the middle meningeal artery (MMA) and distal ECA branches. (h) Left ascending pharyngeal arterial injection shows additional robust supply to tumor. (i) Superselective microcatheter angiography of a tumor feeder from the ascending pharyngeal artery prior to embolization with polyvinyl alcohol (PVA) particles and gelfoam pledgets. Embolization subsequently performed in the MMA and distal ECA (not shown). (j) Final global left ECA run shows marked devascularization of tumor supply from this pedicle. (k) Postoperative CT shows significant debulking of tumor.
Axial and coronal CT images with bone window images will nicely depict the extent to which bone remodeling and erosion is present. Enhanced MRI is helpful in assessing the vascularity and local extension of tumor.15 JNAs are typically well circumscribed despite the propensity for local extension, avidly enhancing, and may demonstrate vascular flow voids. Angiography commonly illustrates the tumor as a high-flow lesion with dense capillary filling and shunting into prominent veins. At times, malignant sino-nasal tumor may mimic a JNA or other benign masses. Careful scrutiny, often involving both CT and MRI, is required for challenging cases. Typically speaking, aggressive lesions possess ill-defined margins, and demonstrate invasion of adjacent tissue and bony destruction, whereas benign masses remodel and expand bone ( Fig. 7.2).16
Fig. 7.2).16
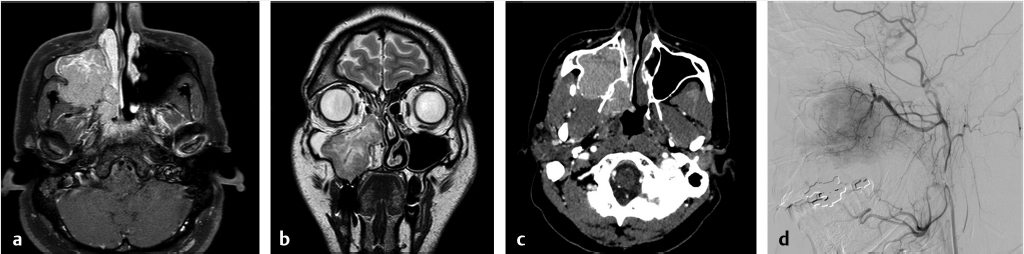
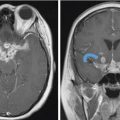
Fig. 7.2 Malignant sinonasal tumor mimicking juvenile nasopharyngeal angiofibroma (JNA). A 37-year-old male with recurrent epistaxis, (a) T1 postgadolinium, (b) coronal T2, and (c) axial computed tomography angiogram images show a large avidly enhancing mass involving the nasal cavity, nasopharynx, and maxillary antrum with extension into the retroantral space. However, the age and lack of antral bowing are atypical. (d) Right external carotid artery injection in preparation for embolization shows much less vascularity than expected for JNA. Embolization was nevertheless performed. Pathological diagnosis was EBV (Epstein-Barr virus) positive plasmablastic lymphoma.
Clinically, JNAs will often appear highly vascular and may present with recurrent (classically unilateral) epistaxis. Due to the vascularity of JNA and risk of catastrophic hemorrhage, image-guided biopsy is to be avoided when the diagnosis is entertained.17 It should be noted that other sinonasal cancers like squamous cell and vascular metastatic disease can simulate the clinical presentation of a JNA through recurrent epistaxis and compressive symptoms.
The blood supply for JNAs typically originates from branches of the internal maxillary artery, namely the sphenopalatine and descending palatine arteries, as well as the ascending pharyngeal arteries and facial arteries. Additional supply can be parasitized from dural branches of the ICA including the meningohypophyseal and inferolateral trunk, and ethmoidal branches off the ophthalmic artery.
Preoperative embolization has the same objective as the other tumors described in this chapter. The goal is reduction of intraoperative bleeding, which may facilitate more comprehensive tumor resection, and shorter recovery. Radiation is another adjunct in circumstances where complete resection is deemed too risky, but has been reported to increase the risk of malignant transformation.
In general, superselective microcatheter embolization of the individual tumor feeders is preferred to arbitrary embolization from a more proximal position even if the latter would encompass all vessels in close proximity to the tumor. In addition to improving the penetration of embolic material into the tumor capillary bed (important for effective embolization), and reducing the chance of inadvertent nontarget embolization through anastomoses, it must be noted that in these tumors, extensive reconstruction of the posterior nasal cavity is frequently required. The superficial temporal and deep temporal arteries provide important vascular supply to the healing soft tissues and to the temporalis muscle flaps which may be used in the reconstruction. Unnecessary embolization of these branches (which might be acceptable in other embolization procedures such as for idiopathic epistaxis) may predispose the patient to suboptimal wound healing or tissue necrosis especially when liquid embolics are used.
Preoperative embolization can shorten operation time, increase intraoperative visibility, and reduce complication rates. Previous systematic analyses have illustrated this point, by estimating the decrease in endoscopic surgical resection blood loss from greater than 800 to around 400 mL after embolization. A similar effect was seen with open surgery, which showed a reduction from greater than 1,900 to less than 700 mL after preoperative devascularization.13 Due to extensive collateral supply to these tumors, there can still be significant residual vascularity despite embolization of the safe pedicles.18 While this may portend more intraoperative bleeding, overaggressive embolization may put the patient at risk of stroke from migration of the embolic agent into the intracranial circulation via the collateral network, and cranial nerve palsies due to unintended occlusion of the vasa nervorum with embolic material.8 This must be weighed against the adjunctive nature of the embolization procedure.
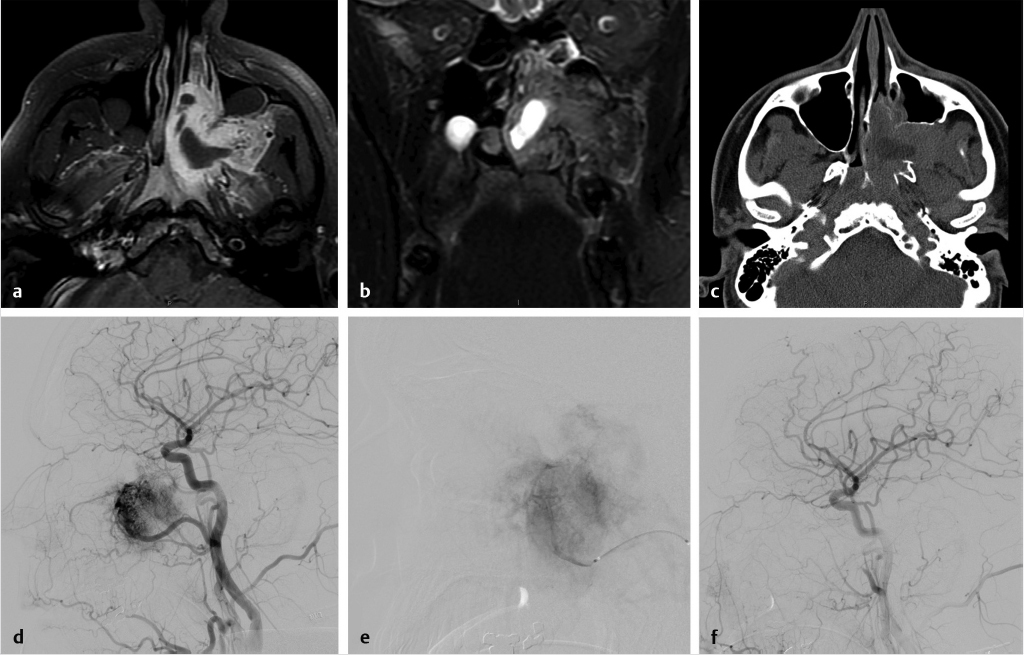
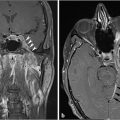
 Fig. 7.3 depicts the pre- and postembolization appearance of a JNA involving the left nasal cavity and masticator space prior to surgical resection. Embolization of PVA particles and gelfoam into the left distal internal maxillary artery resulted in a marked reduction in tumor vascularity.
Fig. 7.3 depicts the pre- and postembolization appearance of a JNA involving the left nasal cavity and masticator space prior to surgical resection. Embolization of PVA particles and gelfoam into the left distal internal maxillary artery resulted in a marked reduction in tumor vascularity.
Where satisfactory transarterial embolization is not feasible for technical reasons, direct puncture techniques followed by injection of liquid agents like NBCA and Onyx have been reported. Ultrasound or CT guidance can be used for more accurate placement of the needle within the tumor while avoiding critical structures.19 The injection is then performed under fluoroscopic or sometimes CT control.
7.4 Paraganglioma
Paraganglioma (PGL), also known as glomus tumor, chemodectoma, and nonchromaffin PGL, originate from neural crest tissue and secrete vasoactive substances including catecholamines and serotonin. They account for 0.6% of all head and neck tumors.20 Adrenergic symptoms should be investigated with urinary VMA and 5-HIAA to test for catecholamines and serotonin, respectively. In general, they present as tumors within the carotid body (at the carotid bifurcation), the jugulotympanic region, or less frequently as vagal tumors or in other locations such as the orbit, nasal cavity, thyroid gland, and sympathetic trunk.21 The most common locations for presentation, in decreasing order are carotid, tympanic, jugular, vagal, laryngeal, orbital, and nasopharyngeal. Some group tympanic and jugular as unified entities termed jugulotympanic or temporal paragangliomas.22 Location is highly correlated with the likelihood of malignant transformation, which has been reported at a range from 2 to 19%.23 Malignant potential is most easily recalled as occurring in the opposite order from rareness, with nasopharyngeal tumors exhibiting the highest likelihood. Many subtypes show equal distribution between males and females; however, the tympanic, jugular, vagal, and nasopharyngeal tumors are more common in females. PGLs have also been associated with other tumors of the amine precursor uptake and decarboxylation (APUD) system such as pituitary adenomas, thyroid carcinomas, and pheochromocytomas. These tumors are typically inherited in an autosomal-dominant fashion with variable expression. Approximately 30% of cases are multicentric diseases, predominantly involving two (84%), followed by three (13%), and four (2%) tumors. Those that have secretory actions and are multicentric have been more closely correlated with a likelihood of malignancy.