A brief introduction is provided of the different imaging modalities encountered in the intensive care unit (ICU). The spectrum of intracranial pathology as well as potential postsurgical complications is reviewed, with a focus on pearls and pitfalls. A brief overview also is provided of imaging of the spine in an ICU patient.
Key points
- •
Attention to quality, safety, utilization, and appropriateness is paramount when evaluating imaging of patients in the intensive care unit (ICU).
- •
Carefully scrutinizing all the provided images in critically ill patients, such as scouts and localizers, is crucial to answering the clinical question.
- •
When evaluating imaging, one must be aware of potential postprocedural complications in the ICU patient.
Brief introduction of imaging modalities
The first step in imaging an ill patient is to determine which modality to choose, if any. Attention to quality, safety, utilization, and appropriateness is paramount. The most pertinent imaging modalities are introduced briefly. Catheter angiography, transcranial sonography, and nuclear medicine brain death scans are not discussed due to space constraints. Diagnostic imaging is also focused on and not invasive procedures, for similar reasons.
Computed Tomography
Computed tomography (CT) is a widely utilized method due to its availability, speed of use, and presence in some ICUs. Noncontrast head CT scans are performed to evaluate patients for urgent intracranial pathologies, such as hemorrhage, herniation, infection/inflammation, infarction, and hydrocephalus. Severe trauma, stroke, and altered mental status are common scenarios where CT may be used.
Computed Tomography Angiography
CT angiography (CTA) uses intravenous contrast with imaging during the arterial phase for detailed evaluation of potential arterial pathology, including aneurysms, vasospasm, infarction, and vascular malformations. CTA of the neck often is performed to evaluate for vascular injuries, including traumatic arterial injury, dissection, transections, and arterial stenosis.
Computed Tomography Venography
CT venography (CTV), like CTA, uses intravenous contrast but instead images the venous phase to evaluate pathologies, such as venous sinus thrombosis, cortical vein thrombosis, and thrombophlebitis. CTV of the neck can be performed to evaluate extent of clot, stenosis, and occlusion. CTV also can be beneficial in the posttraumatic setting to evaluate for injury from blunt or penetrating trauma.
Computed Tomography Perfusion
CT perfusion (CTP) also requires administration of iodinated contrast with dynamic imaging during contrast passage to evaluate for acute ischemia, acute infarction, or vasospasm. Quantitative parameters, including cerebral blood flow, cerebral blood volume, mean transit time, time to peak, and Tmax, are calculated from contrast curves to evaluate for specific pathology.
Iodinated Contrast
Iodinated contrast should only be used when it is required for decisions that affect clinical management. Allergies and impaired renal function can affect their use in ICU patients. The American College of Radiology Manual on Contrast Media and Appropriateness Criteria provide excellent evidence-based resources on when and how contrast is used, which is especially important in ICU patients.
Portable Computed Tomography
Portable CT scanners can increase scan availability and reduce the need to transport critically ill patients, which can be challenging because of the need for portable ventilation, intravenous sedation, and extensive supportive tubes, lines, and drains. Portable CT can be useful in patients too unstable to travel, including those on extracorporeal membrane oxygenation or ventricular assist device. Challenges to interpreting portable CTs include decreased spatial and contrast resolution and increased motion artifact.
Radiographs/Scouts
Use of radiographs is limited to evaluation of lines and tubes, such as a ventricular catheter. Radiographs have advantages, including rapid examination times, ready availability, and low radiation exposure. Scout radiographs, performed as part of CT examinations, also are important for evaluating support apparatus, craniocervical alignment, craniotomies, and surgical hardware.
Magnetic Resonance
Indications for performing magnetic resonance (MR) include further investigating a CT finding, evaluating a patient with persistent neurologic symptoms despite normal CT/prior imaging, and post-treatment follow-up. MR imaging has myriad challenges in ICU patients. MR scanners often are spatially distant from the ICU, requiring stability for transport. Safety-related patient considerations are paramount, and patients require MR safety clearance in regard to devices, implants, foreign bodies, prior surgeries, and so forth. Monitoring equipment must be switched to MR-compatible equipment. Implanted cardiac devices, including pacemakers and defibrillators, pose a special imaging situation, but patients with these devices can sometimes be scanned after consultation with an electrophysiologist and appropriate changes to device settings if local protocols allow for this. On MR images, localizer/scout sequences should be reviewed for any unexpected findings.
Magnetic Resonance Angiography
MR angiography (MRA) is performed to evaluate the arterial vasculature and usually includes the brain and/or neck. Time-of-flight (TOF) technique allows MRA to be performed without the administration of intravenous gadolinium contrast agents. Contrast-enhanced or time-resolved (4-dimensional) MRA may be useful, however, to evaluate specific entities, such as vascular malformations, dissection, and pseudoaneurysm.
Magnetic Resonance Venography
MR venography (MRV) can be performed to evaluate the venous vasculature of the brain and/or neck. Like MRA, MRV can be performed without contrast via the TOF technique or after the administration of gadolinium. TOF MRV is prone to artifacts; therefore, MRV with contrast is preferred to evaluate for dural venous sinus thrombosis.
Gadolinium
As with iodinated contrast, gadolinium agents should be used only when necessary to answer the desired clinical question. Patients’ renal function is a consideration but less of an issue today than years ago. Patients also must be evaluated for potential allergic reactions to gadolinium. Pregnant patients typically are not given gadolinium agents because this has been shown to cross the placenta into the fetus with uncertain effects.
Spectrum of intracranial neurologic pathology
Intracranial Trauma
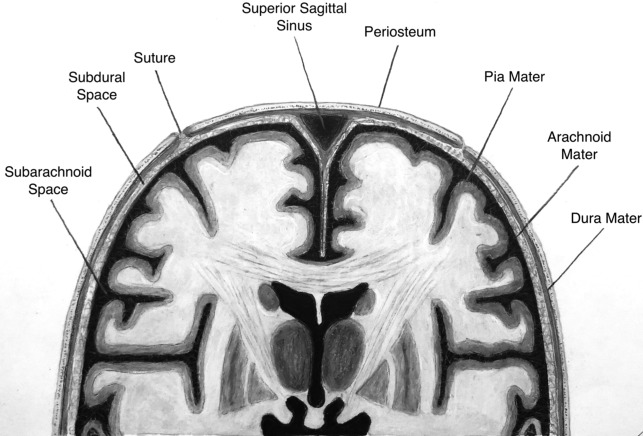
Traumatic injury is a common indication for brain imaging in the ICU setting, with noncontrast head CT usually the first line of imaging. It is crucial to understand the different intracranial compartments in order to understand the types of pathology that can occur ( Fig. 1 ). It is not always possible to place extra-axial hemorrhage into a specific compartment. A subdural hematoma typically is more crescent-shaped and can cross sutures. Subdural hematomas are usually due to tearing of crossing bridging veins. In comparison, epidural hematomas are commonly biconvex or lens-shaped, do not cross sutures, and may cross the midline. Epidural hematomas are associated with calvarial fractures, classically described with middle meningeal artery or midline venous injury.
Trauma is the most common cause of subarachnoid hemorrhage (SAH). When traumatic in etiology, it is typically located in the peripheral cerebral sulci rather than in the sylvian fissures and basal cisterns. Traumatic SAH also may be located adjacent to fractures or cerebral contusions, rather than adjacent to the circle of Willis.
Diffuse axonal injury (DAI) is another entity that may be seen in patients with high-energy trauma and results in altered mental status. These abnormalities usually occur at the grey-white matter junction, corpus callosum, and brainstem, presumably due to differences in mechanical properties at tissue interfaces. CT may show small hemorrhages, although CT is normal in 50% or more cases of DAI. MR imaging, in particular susceptibility-weighted imaging and T2-weighted images, is traditionally more sensitive than CT for DAI. The degree of injury often is underestimated by imaging.
Intracranial Vascular Pathology
ICU patients can have a range of intracranial vascular pathologies, including aneurysm, vascular malformation, ischemia, and vasospasm. When a noncontrast head CT reveals acute intracranial hemorrhage, CTA of the head usually is the most appropriate next examination to evaluate for an underlying vascular lesion, such as ruptured intracranial aneurysm. This hemorrhage is sometimes (although not always) centered around the site of rupture. Other vascular malformations, such as arteriovenous malformations or dural arteriovenous fistulas, can result in single-compartment or multiple-compartment hemorrhage.
Ischemic stroke is another common indication for imaging. This indication may arise in the inpatient setting due to altered mental status or new acute neurologic deficit. Evaluation usually begins with a noncontrast head CT to identify those acute ischemic stroke patients who are candidates for thrombolysis. This noncontrast head CT is used mainly to evaluate for acute intracranial hemorrhage, but this scan also is an opportunity to evaluate for additional potential causes of a patient’s symptoms (eg, metastatic disease and brain tumor) as well as the size, density, and extent of ischemic infarction. If acute intracranial hemorrhage is identified, CTA may be considered to evaluate for an underlying vascular malformation or a site of active bleeding. Depending on institutional preference, stroke patients go on to be evaluated by a combination of CTA and CTP or MR imaging.
Dural venous thrombosis is another pathology that can be seen in ICU patients. The dural venous sinus may appear hyperdense on a noncontrast head CT or there may be concern for a hemorrhagic venous infarction, classically manifested as parenchymal hemorrhages in an unusual location for hemorrhagic transformation of ischemic infarction. Presence and extent of venous thrombosis can be characterized further with CTV or MRV.
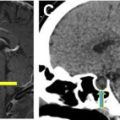
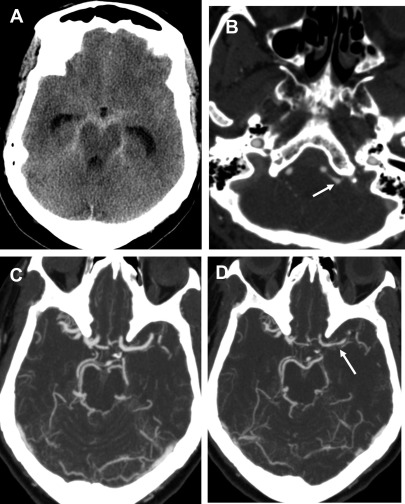
Vasospasm is reactive narrowing of the intracranial arteries, commonly after SAH with peak at 5 days to 14 days after initial hemorrhage. If severe enough, the vasospasm can result in alternations in cerebral perfusion, leading to ischemia/infarction ( Fig. 2 ). Patients usually are evaluated at the bedside with transcranial doppler, but CT/CTA/CTP can be used for further evaluation, often varying with local practice patterns. CTA typically shows interval (that is, recently acquired) intracranial arterial narrowing.
Brain Herniation Patterns and Complications/Low Tonsils
It is crucial to be aware of the various types of brain herniation patterns and their often associated complications ( Fig. 3 ). Subfalcine herniation is characterized by the frontal lobe, commonly the cingulate gyrus, sliding underneath the falx cerebri. Potential complications include compression of the anterior cerebral artery, resulting in acute ischemia or hydrocephalus due to compression at the foramen of Monro.
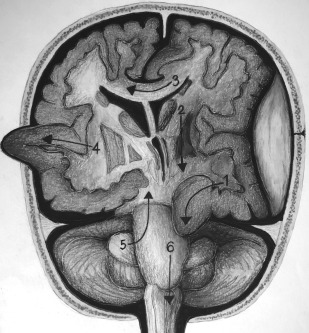
Transtentorial herniation can be unilateral or bilateral, involves brain crossing the tentorium at the level of the incisura/notch, and has several subtypes. Uncal herniation is when the medial portion of the anterior temporal lobe, the uncus, is shifted into the suprasellar cistern. Effacement of the ambient cisterns often can occur, with complications including posterior cerebral artery or brainstem compression. Duret hemorrhages usually are small hemorrhages within the medulla or pons as a result of descending transtentorial herniation. A cerebellar hemisphere crossing the tentorium at the level of the incisura/notch characterizes ascending/upward transtentorial herniation.
Extracranial/transcranial brain herniation can be seen in the setting of trauma with an open calvarial fracture but more commonly (at least in the authors’ experience) is an expected finding after decompressive hemicraniectomy in the setting of increased intracranial pressure (eg, after middle cerebral artery stroke).
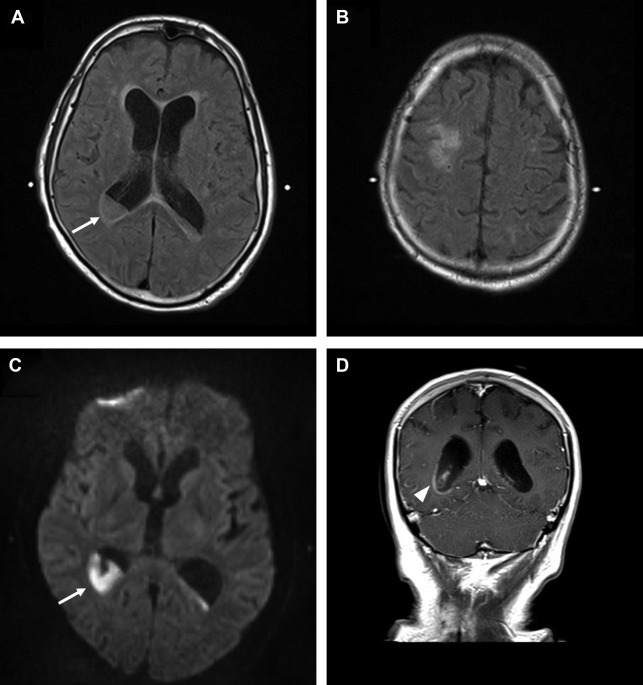
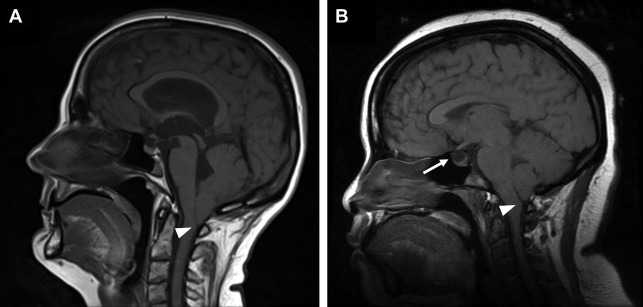
Cerebellar tonsillar herniation is manifested as crowding at the level of the foramen magnum due to descent of the cerebellar tonsils. Low-lying cerebellar tonsils extending through the foramen magnum are not synonymous with acute brain herniation, because there are other potential causes of this finding including Chiari I malformation, idiopathic intracranial hypertension, and cerebrospinal fluid (CSF) hypotension ( Fig. 4 ).
Hydrocephalus
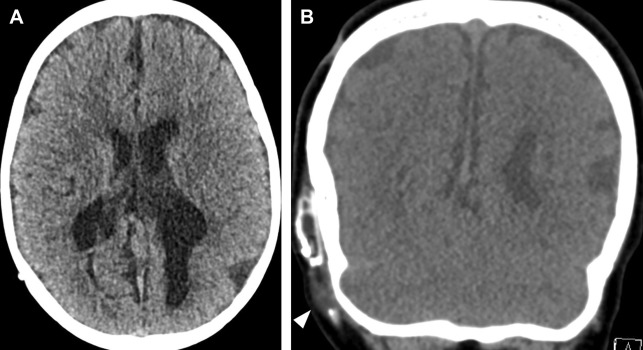
Hydrocephalus is active distension of the ventricular system due to inadequate CSF flow from its origin to the point of absorption. Hydrocephalus can be communicating or noncommunicating/obstructive and acute or nonacute. Communicating hydrocephalus demonstrates enlargement of all ventricles, whereas noncommunicating hydrocephalus involves only ventricles proximal to a point of obstruction within the ventricular system. Acute or uncompensated hydrocephalus is characterized by periventricular edema on CT and MR. Noncontrast head CT is the usual initial imaging modality in patients with known or suspected hydrocephalus. A short brain MR imaging is an alternative used at some institutions, especially pediatric hospitals to evaluate the ventricular size in patients with hydrocephalus. These studies typically consist of a fast T2-weighted sequence (such as a T2 HASTE) in 1 or more planes as well as a diffusion-weighted sequence, depending on the institution. For patients with a shunt, the visualized portions of shunt hardware on CT and MR imaging should be scrutinized carefully for discontinuities or unexpected abnormalities ( Fig. 5 ).
Intracranial Infections/Inflammatory
Intracranial infections, including meningitis, encephalitis, and focal abscess, can be challenging to diagnose and are a significant concern in the ICU patient. These all can cause changes in mental status, which can lead to hospitalization in the ICU or develop in patients already hospitalized for other reasons.
Meningitis can occur spontaneously or be iatrogenic, such as after an intracranial surgery or spinal procedure. When there is suspicion for meningitis, initial imaging typically is performed with CT of the head without contrast, which, despite low sensitivity for intracranial infection, is useful for excluding masses and hemorrhage and assessing for mass effect. In some cases of infection, edema may be visualized on CT. MR imaging of the brain with contrast is the mainstay of imaging for intracranial infection.
Imaging findings of meningitis include leptomeningeal enhancement, brain swelling, hydrocephalus, and altered CSF intensity on T1 and fluid-attenuated inversion recovery (FLAIR). In severe cases, CSF can be T1 hyperintense and fail to suppress on FLAIR, commonly in the prepontine cistern and cerebral sulci. Meningitis can progress to frank involvement of CSF within the ventricles or ventriculitis. Other ominous signs include FLAIR hyperintensity, restricted diffusion (implying frank pus), and periventricular edema or enhancement ( Fig. 6 ). Abnormal appearance of the CSF also can be artifactual, particularly when patients are on supplemental oxygen. Performing FLAIR postcontrast can increase sensitivity for leptomeningeal disease. Basal meningitis, when abnormal imaging enhancement is centered on the basal cisterns, prepontine cisterns, and sylvian fissures ( Fig. 7 ), suggests unusual causes, such as tuberculosis, fungi, or sarcoidosis.