CHAPTER 20 Neurointerventions
Anatomic and technical considerations
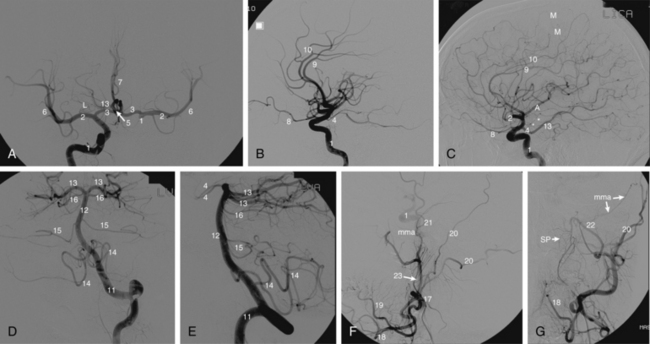
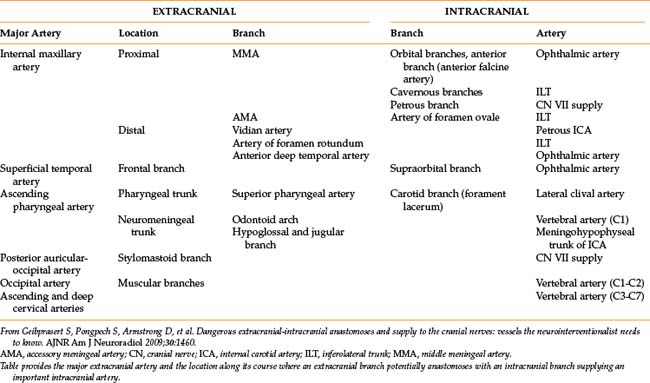
Multiple sources describe the angiographic anatomy of the cerebrovascular system1,2 (Fig. 20-1). Interestingly, large portions of the anterior (carotid) and posterior (vertebrobasilar) circulations supply areas of the brain in which little is understood regarding functionality. In effect, one may occlude a large vessel as seen by angiography, resulting in essentially no clinical neurologic change. Alternatively very small vessels may supply highly important areas where vessel occlusion may manifest as major neurologic deficits. In addition, whenever an artery is occluded, the clinical neurologic result not only depends upon the functionality and degree of cerebral tissue supplied, but also the presence and degree of collateral circulation. These collaterals can at times be troublesome, particularly during external carotid embolization, where they may supply the intracranial circulation3 (Table 20-1).
As in the peripheral circulation, all embolic agents are carefully chosen based on the disease process, and are individualized for a particular patient and his or her unique clinical situation. Coils and particles of various sizes are the most often used. Pushable standard coils (0.035 and 0.038 inch) are used to occlude large vessels such as the vertebral or carotid arteries, as well as larger branches of the external carotid artery (ECA). However, most neurointerventionalists are more comfortable with the precision and safety of electrolytically or mechanically detached coils.4 Now that detachable balloons are no longer available in the United States, occlusive plugs are gaining favor for rapid occlusion of large vessels.5 Particulate agents (polyvinyl alcohol [PVA] and gelatin microspheres) are used for small artery occlusion, such as in epistaxis or preoperative tumor embolization.6 If the operator can place the microcatheter close to the tumor bed, particles of very small size (e.g., 50 to 100 microns) are appropriate. However, larger particles are also used if there is particular concern for nontarget embolization, especially of neural tissue or skin. Liquid agents such as cyanoacrylate, ethylene vinyl copolymer (Onyx Liquid Embolic System, eV3 Neurovascular, Irvine, Calif.), and sclerosants are more commonly used extracranially for facial arteriovenous malformations.7
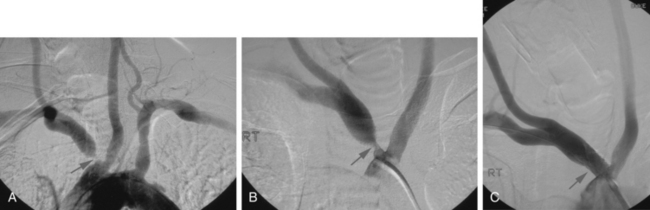
Angioplasty and stenting of the extracranial internal carotid artery is carried out using embolic protection devices to prevent embolization to the intracranial circulation. Embolic protection involves either deployment of a filter beyond (above) the lesion or inflation of proximal/distal endovascular balloon devices during the revascularization process. Filters consist of a basket mounted on a special 0.014-inch guidewire (Fig. 20-2). The mesh is calibrated to be small enough to trap emboli but large enough to permit normal blood flow through the basket. The device serves as the guide for balloon and stent placement. The filter is gently guided through the lesion and deployed in a normal, relatively straight segment of the internal carotid artery above the lesion. Once angioplasty and stenting is completed, angiography is performed to determine whether embolic material has been captured. If present, removal by suctioning is required, and specialized catheters are available to perform this task. Once the distal protection device is angiographically free of debris, it is collapsed and removed using a special catheter specific to each device. Although distal protection is now standard of practice and required by many payers in the United States, the data supporting this approach are controversial.8
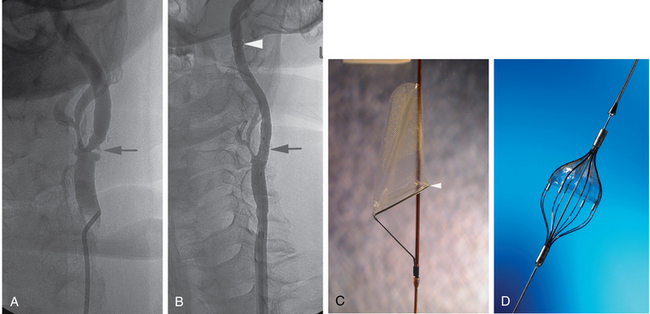
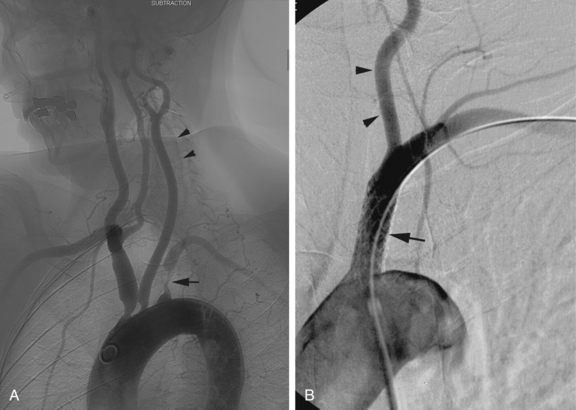
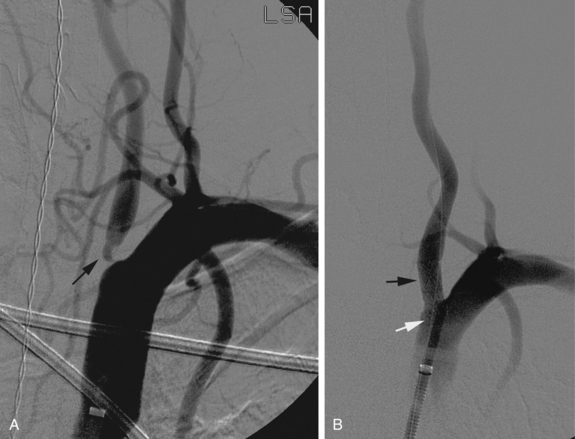
Figure 20-2 Cervical carotid artery angioplasty and stenting in a 74-year-old man with occluded right internal carotid artery and prior left cerebral transient ischemic attack. A, Anteroposterior view of left carotid bifurcation demonstrates a 60% narrowing of the proximal internal carotid artery (arrow) with ulceration and narrowing of the proximal external carotid artery as well. B, Anterior posterior angiogram of the left carotid bifurcation after angioplasty and stent placement. No residual stenosis is noted at the proximal internal carotid artery (arrow). Note the distal protection device (arrowhead) in a relatively straight segment of the internal carotid artery. C, FilterWire EZ distal protection device used in the patient shown in B. Note that the protection device is part of the guidewire and the mesh is radiolucent but fixed to a radiopaque ring inferiorly (arrowhead).D, Angioguard Emboli Capture Guidewire. Note that there are a number of designs for distal protection devices.
(C,Courtesy of Boston Scientific, Natick, Mass., with permission.D,Courtesy of Cordis, Miami Lakes, Fla., with permission.)
Extracranial atherosclerotic disease
Etiology and natural history
Atherosclerotic disease of the extracranial and intracranial carotid and vertebral arteries and their branches clearly place patients at risk for embolic stroke. The long-term natural history of arterial stenoses at the origins of the great vessels is essentially unknown as opposed to the carotid bifurcation where up to 60% of asymptomatic patients with a greater than 75% stenosis have symptoms within 1 year of diagnosis.9
Clinical features
Treatment of atherosclerotic lesions in these vessels demands that the patient’s symptoms correspond to the vascular territory supplied by the particular vessel (vertebrobasilar or carotid system). A thorough and unbiased neurologic examination must be performed and documented before any intervention is undertaken. Although carotid bruits have limited value for the diagnosis of carotid artery stenosis, they are good markers of generalized atherosclerosis, and their absence may constitute sufficient screening of the asymptomatic patient.10 Because detection of a bruit depends on blood flow, it may actually disappear in the setting of a critical stenosis.
Imaging
Diagnostic imaging entails magnetic resonance (MR) or computed tomography (CT) angiography and sonography limited to the cervical common carotid artery (CCA) and internal carotid artery (ICA). Catheter angiography is usually reserved for patients who require some intervention. Rarely, a catheter angiogram is necessary to confirm or clarify findings in a patient not destined for direct treatment. As a general rule, symptomatic carotid artery stenoses greater than 50% of the lumen diameter and asymptomatic lesions of greater than 70% are considered for surgical or endovascular treatment.11–13
Treatment
Medical therapy
Current medical therapy alone may be as good as surgery in asymptomatic patients with carotid bifurcation disease. This approach is estimated to be at least three to eight times more cost-effective than direct intervention in the prevention of stroke.14
Surgical therapy
Fortunately, the sites of typical disease in the extracranial carotid artery are readily accessible to the surgeon for carotid endarterectomy (CEA). The indications for CEA are based on the degree of arterial stenosis, presence of associated symptoms, and suitability for surgery. Current evidence to support CEA comes largely from two randomized trials: the North American Symptomatic Carotid Endarterectomy Trial (NASCET) and the Asymptomatic Carotid Atherosclerosis Study (ACAS).11,12 NASCET proved that, compared with available medical therapy, CEA significantly reduced the risk for stroke in symptomatic patients. However, the surgical results were much more modest for asymptomatic patients, reducing the risk for stroke from 2% to 1% per year.
Endovascular therapy
Reports of successful balloon angioplasty for atherosclerotic disease of the brachiocephalic arteries, including the internal carotid artery, were first reported in the 1980s.15 Balloon dilation continued to gain in popularity at anatomic sites that were surgically less accessible, including the subclavian and vertebral arteries. However, little was done endovascularly for carotid artery bifurcation disease because of fears, perceived and real, that an irregular lumen or abrupt closure would be the angioplasty result.16,17 With the introduction of stents in the mid-1990s, these perceived problems were largely solved, with the pressing issue becoming the outcome differences between surgery and stenting.18
Patient selection and preparation
Angioplasty and stenting of the cervical carotid artery has been the subject of extensive study. Until recently these procedures were restricted to patients who were considered at high-risk for surgery.19 However, there is now approval from the U.S. Food and Drug Administration for all patients with significant cervical carotid artery stenoses regardless of symptomatology or suitability for surgery (Box 20-1). For the great vessel origins and the extracranial vertebral artery, endovascular techniques are often preferred over operation.
Box 20-1 High-Risk Criteria for Carotid Endarterectomy
Anatomic
FEV1, forced expiratory volume in 1 second; LVEF, left ventricular ejection fraction.
All risks are relative and somewhat dependent on the particular patient and surgeon.
From Yadav JS, Wholey MH, Kuntz RE, et al. Protected carotid-artery stenting versus endarterectomy in high-risk patients. N Engl J Med 2004;351:1493.
Before angioplasty and stenting of the cephalic vessels, antiplatelet and anticoagulation therapy is essential. Experimental and clinical data from animal models and human coronary intervention suggest that aspirin and clopidogrel (Plavix) have a synergistic effect on inhibition of platelet aggregation, antithrombotic activity, and prevention of myointimal proliferation.20,21 Therefore patients receive a dual antiplatelet regimen consisting of aspirin (81 or 325 mg daily) and clopidogrel (75 mg daily). This treatment is started at least 5 days before the procedure or given as a loading dose of aspirin (325 to 700 mg) and clopidogrel (300 to 600 mg) early on the day of the procedure. This combination therapy is maintained for 1 month after stenting; one antiplatelet agent (usually aspirin) is continued without interruption indefinitely. For the procedure itself, heparin (or a direct thrombin inhibitor in allergic patients) is administered to achieve an activated clotting time of at least twice what is normal (e.g., 250 to 300 seconds.)
Contraindications specific to carotid and vertebral artery stenting are relative and include inability to tolerate antiplatelet therapy and recent stroke. The latter exclusion criterion is based on experience with CEA, where revascularizing the inflow to areas of acutely infarcted cerebral tissue may result in intracranial hemorrhage.22
Technique and results
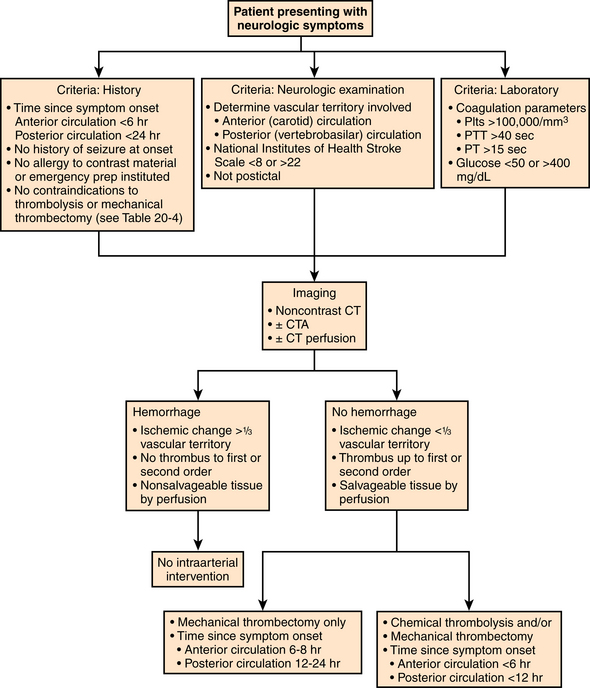
Atherosclerotic lesions involving the great vessels often occur at or near their origins and are therefore most often treated using stents.23,24 Angioplasty alone may be undertaken in the midportions of these vessels but lesions located at the carotid bifurcation are currently relegated to stenting. Balloon-expandable stents are often preferred to allow accurate device placement at the origin of the vessel (Fig. 20-3). Fortunately, balloon-expandable stents are generally not subject to extrinsic compression and collapse at these locations deep in the chest. Self-expandable systems are a reasonable alternative, particularly if vessel tortuosity or variations in vessel diameter are encountered. The use of distal protection devices for proximal great vessel angioplasty is based on operator preference. The results of great vessel angioplasty for atherosclerotic lesions are excellent.25–27 Primary technical success ranges from 93% to 99% with cerebral ischemic complications ranging from 2% to 3%. Long-term patency ranges from 93% at 1 year to 79% at 5 years.
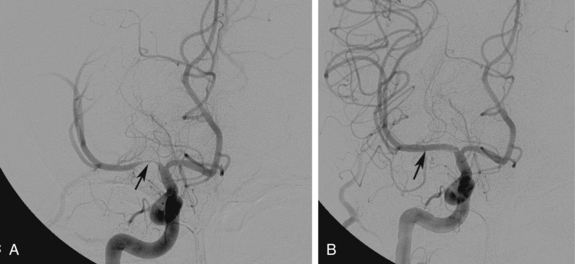
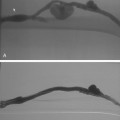
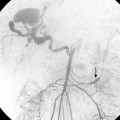
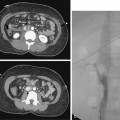
The term subclavian steal is used when a significant stenosis or occlusion of the subclavian artery proximal to the vertebral artery origin occurs (Fig. 20-4). Blood from the contralateral vertebral artery is “stolen” from the posterior fossa via the basilar artery then coursing down the ipsilateral vertebral artery to supply the arm. However, simple reversal of blood flow in a vertebral artery has been found in 6% of patients undergoing carotid ultrasound, far more often on the left than the right side (82%).28 The diagnosis of subclavian steal syndrome is reserved for patients who are symptomatic from resulting posterior fossa ischemia, typically complaining of dizziness or ataxia. Angioplasty and stenting have achieved widespread acceptance as the procedure of choice in this situation. Technical success exceeds 95% for stenotic lesions; for total obstructions, success rates fall to 65% to 85%, largely from failure to traverse the occlusion.15–17 The 5-year clinical patency rates range between 82% and 89%.29,30 Protection devices are rarely used in the vertebral artery during subclavian artery angioplasty and stenting based on the concept that the brain is protected from microemboli by the flow reversal in the ipsilateral vertebral artery.31 Nonetheless, strokes have been reported.30 Stenting across the vertebral artery origin has been undertaken, particularly when symptoms consist of arm claudication and the ipsilateral vertebral artery is the nondominant vertebral vessel.
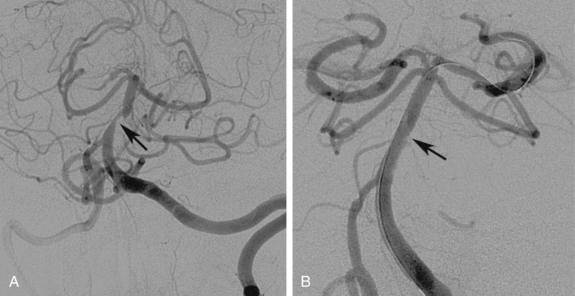
Cervical vertebral artery atherosclerotic disease is prevalent in patients with peripheral arterial disease. However, the diagnosis is often difficult owing to the relative vagueness of symptoms. It may even be difficult to determine whether patient complaints are related to a vertebral artery stenosis or occlusion if the contralateral vertebral artery is patent or the ipsilateral collateral circulation is abundant. Once a connection is made between symptoms and vertebral artery obstruction, angioplasty itself is straightforward and produces acceptable results. Angioplasty with stent placement is performed for lesions at the orifice of the vertebral artery but angioplasty alone may be otherwise applied through the extracranial vertebral artery (Fig. 20-5). Procedural and clinical success has been reported to be 100% and 90.5%, respectively, with 79.3% of individuals remaining symptom-free at 1 year.32 Only one small randomized study of vertebral artery angioplasty has been carried out, which included 16 patients with vertebral artery disease, eight treated with medical therapy and eight by endovascular stenting.33 At a mean of nearly 5 years, neither group had strokes referable to the vertebral artery distribution. A Cochrane database review in 2005 identified 173 reports of vertebral artery stenting and found a 30-day major stroke and death rate of 3.2% and a 30-day transient ischemia and nondisabling stroke rate of 3.2%.34
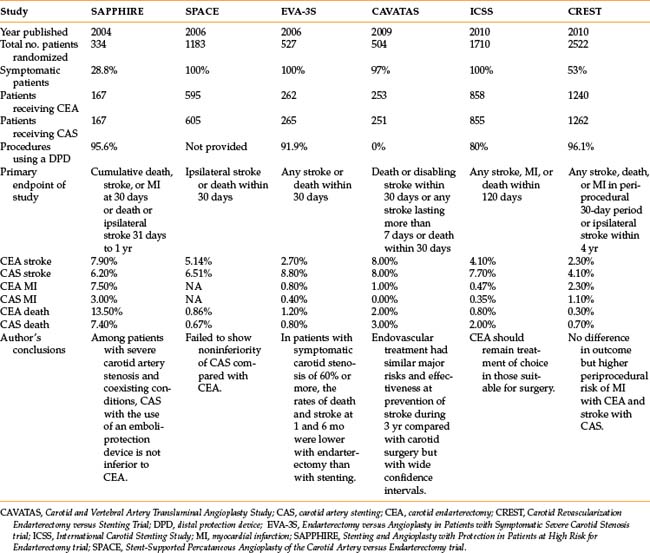
The published results for angioplasty and stenting of the carotid artery bifurcation are mixed.19,35–39Table 20-2 presents the six clinical trials in which carotid artery stenting (CAS) was randomized to carotid endarterectomy (CEA).19,35–39 The studies varied widely in terms of inclusion criteria, symptoms, endpoints, qualifications of the operators, and standardization of techniques. Out of five studies, three found that CAS was not inferior to CEA while two found CAS inferior. Thus far, the best study comparing CAS with CEA in surgically suitable patients is the Carotid Revascularization Endarterectomy versus Stenting Trial (CREST) published in 2010.35 This randomized trial included 2502 patients, both symptomatic and asymptomatic, followed for a median of 2 years after treatment for the primary endpoints of stroke, myocardial infarction, and death. There was overall no difference between the two groups. However, in the 30-day periprocedural period, stroke occurred more often in the stenting group (CEA 2.3% vs. CAS 4.1%) whereas myocardial infarction was greater in the surgery group (2.3% vs. 1.1%) (see Table 20-2). The estimated 4-year rates for the primary endpoints were equivalent.
Complications
Stroke is the most dreaded complication when treating any vessel supplying cerebral tissue. For the carotid artery bifurcation, ischemic stroke ranges from 4.1% to 8.8% in the perioperative period for carotid artery stenting (see Table 20-2). Other complications, however, may occur. Hypotension and bradycardia following carotid artery stenting have been reported in up to 35% of patients because of stretching of the baroreceptors located in the carotid sinus.40 These receptors operate through the autonomic nervous system and are an extremely important regulatory mechanism in the short-term control of blood pressure and heart rate. After CAS, stretching of these baroreceptors by the stent may induce dysfunction leading to hemodynamic instability.41 For this reason, patients undergoing CAS are often pretreated with atropine (up to 1 mg) and the interventional team must be ready to treat hypotension and bradycardia aggressively. Such cardiovascular changes may be very short-lived (minutes), or alternatively, vasopressive agents may be necessary until the baroreceptors autoregulate back to baseline. Patients older than 80 years have a greater incidence of neurologic symptoms following CAS than do the same aged-patients after CEA. These elderly individuals may have a greater propensity for symptomatic emboli in the face of a lessened cerebral reserve.42
The hyperperfusion syndrome is a rare but potentially lethal complication after endovascular or open carotid revascularization. It has been defined as a neurologic deficit or seizure ipsilateral to the treated carotid artery related to chronic ischemia rather than cerebral embolism. Complete recovery is the rule in mild cases, but disability and death can occur in more severe cases.
Intracranial atherosclerotic disease
Natural history
Atherosclerosis of the major intracranial arteries accounts for an estimated 8% to 10% of ischemic strokes in the United States.43 Furthermore, among patients with a 70% to 99% stenosis and symptoms (transient ischemic attacks or stroke), a second ischemic event occurs within 1 year in the territory of the symptomatic artery in 11% of individuals.44 Although the actual prevalence of intracranial atherosclerotic disease is largely unknown, it is more common in African Americans and Hispanics, as well as patients with insulin-resistant diabetes and those with hypercholesterolemia and inflammation.45,46
Treatment
Medical and surgical therapy
Surgery for intracranial atherosclerosis was evaluated in the 1985 external carotid to internal carotid bypass study, which concluded that bypass failed to reduce the risk of ischemic stroke compared with medical therapy.47 Although interest has revived recently, surgery is largely reserved for patients who have failed medical therapy and who are not candidates for or have failed endovascular interventions.
Based for the most part on the failure of surgical bypass, medical therapy for intracranial atherosclerosis has focused on the use of warfarin. However, the results of the Warfarin-Aspirin Symptomatic Intracranial Disease study (WASID) demonstrated that warfarin was associated with significantly higher rates of adverse events and provided no benefit over aspirin.48 Antiplatelet agents, including aspirin and clopidogrel, are currently the primary drugs used in the medical treatment of intracranial atherosclerotic disease.
Endovascular therapy
Endovascular therapy for intracranial atherosclerosis has been limited to symptomatic patients with greater than 50% stenosis who have failed medical therapy and asymptomatic patients with stenoses who are counseled regarding the therapeutic options and a wish to proceed with recanalization.49 To lessen the likelihood of vessel perforation, intracranial endovascular therapy should be limited to the major proximal vessels (intracranial carotid and vertebral, basilar, M1, and rarely P1; see Fig. 20-1). Autopsy data have noted that the distribution of intracranial atherosclerotic disease largely involves these same vessels.50
Intracranial angioplasty procedures are typically (though not universally) performed with general anesthesia. The therapeutic options are angioplasty alone or angioplasty with stenting. It is usually easier to negotiate the tortuous vertebral and carotid vessels with a balloon alone; advancing a stent through these pathways can be quite difficult. Stenting has thus far not been shown to be superior to angioplasty alone and are thus usually grouped together in publications.51 Angioplasty alone carries the fears of an intimal dissection, some advocating that the occurrence of a significant intimal flap can be limited by slow, prolonged balloon inflations (approximately 2 minutes) as opposed to quick, sudden inflations.52 Stents can be either of the self-expanding or balloon-expandable variety (Figs. 20-6 and 20-7). Balloon-expandable stents are for the most part coronary stents used in an off-label fashion. A single self-expanding stent (Wingspan Stent System, Boston Scientific, Natick, Mass.) has a human device exemption from the U.S. Food and Drug Administration (FDA) for intracranial use (see Fig. 20-6).
Data regarding intracranial angioplasty and stenting are now being published.53 A systematic outcomes review in 2009 identified 31 studies reporting on 1177 procedures, performed mostly (98%) in symptomatic patients with high-grade (mean 79%) intracranial stenoses.54 There was a high technical success rate (median 96%) with periprocedural minor or major stroke and death rates ranging from 0% to 50% (median 7.7%). Periprocedural complications were significantly higher in the posterior versus the anterior circulation, but did not differ between patients treated with balloon-mounted versus self-expanding stents. However, restenosis greater than 50% at a mean of 8.7 months occurred more frequently after the use of self-expanding stents.
Two multicenter prospective nonrandomized patient registries have been conducted by product manufacturers resulting in human device exemption status from the FDA: the Stenting of Symptomatic Atherosclerotic Lesions in the Vertebral or Intracranial Artery (SSYLVIA) study and the Wingspan stent system with Gateway PTA Dilation Catheter (Boston Scientific, Fremont, Calif.).55,56 The SSYLVIA trial used the Neurolink System, a self-expanding stent (then Guidant, Menlo Park, Calif.), and found an overall symptomatic restenosis rate of 13.7% with an initial procedural success rate of 85.5%.55 The Wingspan study had a procedural success rate of 98%, although a 7.5% greater than 50% restenosis rate at 6 months.56 The Wingspan self-expanding stent is available under a human device exemption; the Neurolink stent is not currently available. A randomized study between the Wingspan stent and best medical therapy was undertaken in the Stenting and Aggressive Medical Management for Preventing Recurrent Stroke and Intracranial Stenosis trial (SAMMPRIS). This large study compared medical to endovascular therapy in symptomatic patients with a 70% to 99% stenosis of a major intracranial artery. The study was recently stopped due to a high rate of complications in the stenting group. Publication of the data is pending at this writing.
Acute stroke
Etiology and natural history
By definition, stroke refers to a fixed neurologic deficit of greater than 24 hours duration. The estimated incidence of stroke in the United States is approximately 795,000 per year. Stroke is the third leading cause of death in the United States and the leading cause of long-term disability.57 Eighty-three percent of strokes are ischemic and related to large artery atherosclerosis, thromboembolism, small vessel occlusion, or other causes.58 The remaining 17% of cases are hemorrhagic (about 10% intracerebral and 7% subarachnoid).
Stroke is the clinical situation that follows neurologic cell death because of decreased blood flow. However, acute stroke has a large component of fluctuating ischemia. The ischemic penumbra refers to the region of threatened tissue adjacent to the core of evolving infarction. It is thought that this area has a limited and variable interval of viability and is potentially salvageable by the quick restoration of blood flow. The primary goal of acute stroke treatment is preventing frank infarction in this fragile region.
Clinical features
The patient should be expediently evaluated by the neurologic stroke team for findings of ischemic stroke (Fig. 20-8). A complete history is obtained, sometimes from a family member. The presence of contraindications to the administration of intravenous thrombolytic therapy is determined (Box 20-2). A key item in the history is the exact time of symptom onset (or time the patient was last known to be in his usual state of health).
Box 20-2 Intravenous Recombinant Tissue Plasminogen Activator for Acute Ischemic Stroke*
Contraindications to administration
The administration of intravenous t-PA is not recommended in the following cases:
Generally accepted guidelines for administration
Indications
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree