Agent
Strength
Unique property
Negative properties
Systemic toxicity
Alcohol
50–100 %
Hypobaric, fast onset
Painful on injection, risk of neuritis (peripheral nerves)
Disulfiram-like reaction
Phenol
4–15 %
Hyperbaric, painless, slow onset
Shorter-lived, affinity for vasculature
Convulsions, cardiovascular collapse
Glycerol
50 %
Historically applied to Gasserian ganglion for treatment of trigeminal neuralgia
Inability to control the spread
Severe headache or local dysesthesia
Capsaicin
8 %
Nociceptor-selective, topical
Painful application, only for localized neuropathic pain
Ammonium salts
10 %
Sensory fiber-selective, motor intact, lack of postblock neuritis
Nausea and vomiting, headache and paresthesia
Nausea and vomiting, headache and paresthesia
Phenol
Protein coagulation causes nonselective tissue destruction and initiates Wallerian degeneration in nerves [15].
Intrathecal administration causes degeneration of large and small nerve fibers within the nerve roots but not the ganglia or spinal cord [17].
It has affinity for vasculature and is toxic to vasculature [15]; therefore, use caution in vascular locations (celiac plexus).
Neurolysis lasts for several months [18]; regeneration is more rapid than alcohol (14 weeks) [19]. Milder blockade (compared to alcohol).
Concentrations <−2 % act as a local anesthetic and >5 % needed for neurolysis [18].
Mixing possible with water or saline up to 6.67 %, mixing with glycerin for higher concentrations, and mixing with radiopaque dye possible.
Not painful on injection.
Hyperbaric compared to CSF (especially when mixed with glycerol); position patients with the painful side down during intrathecal injection in order to coat the dorsal roots.
Phenol diffuses out of glycerin slowly; there is time for patient positioning after injection.
Glycerin’s high viscosity requires at least a 20-gauge needle for injection.
Alcohol
Induces Wallerian degeneration in peripheral nerves [20].
Subarachnoid application causes Wallerian degeneration, demyelination, and degeneration in the dorsal roots, posterior columns, and dorsolateral tract of Lissauer [16].
Neuronal regeneration in peripheral nerves and spinal cord begins after 1–3 months [16].
Concentrations up to 33 % destroy sensory nerves but spare motor nerves when applied peripherally.
Concentrations >33 % may cause paralysis.
Concentrations 50–100 % are used intrathecally.
40 % alcohol is equipotent to 5 % phenol.
Available in the United States in a 95 % concentration in 5-ml vials.
Rapidly spreads from the injection site
Requires larger volumes (than phenol due to rapid spread)
Easily absorbed into the bloodstream [21], peak blood alcohol levels after injection are usually below the legal limit for driving unless accidental intravascular injection has occurred.
Hypobaric compared to CSF.
When performing intrathecal neurolysis, the patient must be positioned with the painful side up in order to coat the appropriate dorsal roots.
Alcohol is painful on injection.
Give local anesthetic prior to application to peripheral nerves.
Intrathecal application less painful than peripheral, with only transient mild burning.
Toxic to vasculature, causes vasospasm and possibly thrombosis [15].
Toxic to connective tissue, causes necrosis.
Inject small volumes and flush the needle with sterile saline prior to withdrawal [19].
Glycerol
The mechanism of action is unclear, but it appears to cause Wallerian degeneration [19].
Historically most commonly used to treat trigeminal neuralgia but newer options including radiofrequency lesioning are replacing the use of glycerol.
Rarely used to treat cancer pain.
Concentration of 50 %.
Ammonium Compounds
Hypertonic Solutions
Serious complications including death secondary to hypertonic saline make them clinically undesirable [26].
Hypertonic saline 10 % NaCl has been used in percutaneous epidural neuroplasty for the treatment of radiculopathy and low back pain.
Intrathecal hypertonic saline (10–15 %) has been shown to decrease pain by 50 % in cancer patients for up to 3 months [27].
Vanilloids
Capsaicin (active ingredient in hot chili peppers) and resiniferatoxin (RTX) are available for use.
Desensitize unmyelinated C pain fibers.
Activate the transient receptor potential vanilloid receptor 1 (TRPV1) on unmyelinated C fiber nociceptors; influx of calcium and sodium ions depolarize the nociceptive afferent terminals; release of stored neuropeptides causes initial pain signaling followed by desensitization [28].
Early desensitization of nociceptors by conduction block.
Delayed desensitization by downregulating TRPV1 receptors [29].
TRPV1 receptors have been identified in visceral organs, spinal cord, and DRG [29].
Selective for nociceptors.
Speed of onset and duration of analgesia depends on dose, duration, and frequency of exposure [30].
Effect is temporary, lasting hours to days, and requires frequent reapplication to maintain effect.
Topical application several times daily.
The topical application of low-dose capsaicin (<1 %); an effective adjunct to the treatment of postherpetic neuralgia, postmastectomy pain, and diabetic neuropathy [31].
NGX-4010 (8 % capsaicin patch) provides pain relief for up to 12 weeks after one 60-min application [32].
NGX-4010 is proven effective in the treatment of HIV-associated distal sensory polyneuropathy [33, 34] and postherpetic neuralgia [32].
Intrathecal resiniferatoxin has been shown to reduce pain in a canine bone cancer model [35]. Phase I clinical trials in cancer patients will start soon.
Clostridial Neurotoxin
Botulinum toxin is neurotoxic to cholinergic nerves by blocking acetylcholine release.
Analgesic effect also secondary to inhibition of calcitonin gene-related peptide (CGRP) release from afferent nerve terminals [36], substance P from dorsal root ganglia, and glutamate in the dorsal horn [37].
Botulinum toxin (BTX) has seven serotypes (A–G) which consist of a heavy chain bound to a light chain by a disulfide bond [38]. The heavy chain binds the nerve terminal and facilitates internalization of the light chain. The light chain internally inhibits neurotransmitter vesicle docking on the plasma membrane [38].
Neurotransmitter vesicular docking is mediated by the soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) complex which is the target for the BTX light chain.
Normal nerve terminal function eventually recovers through restoration of the SNARE complex [37],
Clinically, motor paresis develops within 5 days and lasts for several months.
BTX is too large to penetrate the blood-brain barrier and is inactivated by retrograde axonal transport; therefore, there is no direct central nervous system effect [37]. Effective in treatment of painful muscle spasticity and myofacial pain, hyperhidrosis, hypersalivtion, and hyperlacrimation [38].
In cancer patients, BTX has been shown to improve symptoms of radiation fibrosis syndrome [39] and neuropathic pain [40]. Currently approved for use in the United States are Onabotulinumtoxin A (Botox/Botox Cosmetic), Abobotulinumtoxin A (Dysport), and Rimabotulinumtoxin B (Myobloc).
The dose equivalency is 20 U vs. 50 U vs. 2,000 U for Botox, Dysport, and Myobloc, respectively.
BTX is injected into striated muscle in increments of units.
Dosage units differ among the BTX products and are not comparable or convertible [41].
BTX may be diluted in local anesthetic or sterile saline, and optimal dilutions have not been established for treatment of pain.
Smaller effect or shorter duration of response seen over time due to development of antibodies against BTX [37].
If antibodies are suspected, rotation to different serotype usually effective.
Local complications include muscle atrophy, dysphagia, dysphonia, and ptosis.
Systemic complications include dyspnea, respiratory compromise, weakness, and death. Systemic complications have mostly occurred in children treated for cerebral palsy-associated spasticity and have been reported between 1 day and several weeks following treatment [42].
Clinical Practice
The following clinical examples are the approaches the authors of this chapter have found most successful for neurolysis. These clinical descriptions are intended for example only and should be interpreted for use by experienced clinicians. We support the use of the following neurolytic agents for palliative pain control in patients with malignant pain. We urge caution when using these neurolytics in patients with non-cancer pain or those with normal life expectancy due to potential for permanent catastrophic complications. We endorse the use of imaging when possible for these procedures. We also recommend avoiding heavy sedation of the patient. Patients should remain alert enough to report symptoms suggestive of any complication.
While specific complication risks are mentioned below, complications may result from any aspect of neurolysis from technical procedural complications to agent-specific complications. Technical complications include infection, bleeding, perforation of a viscus or organ, pneumothorax, unintentional subarachnoid or epidural injection, vascular laceration or injection, and peripheral nerve trauma. Complications from the neurolytic agent include motor block, paraplegia, neuropathic pain and dysesthesias, skin ulceration, soft tissue and muscle injury, phlebitis, thrombosis, and tissue ischemia [15]. Although less devastating, neuralgias, hypesthesia, and anesthesia following neurolysis may be very distressing to patients expecting relief of suffering. These complications are more common following traditional neurolytic agents such as alcohol. Finally, neurolysis does not always provide complete neuronal blockade. The neurolytic block often clinically provides less analgesia than the local anesthetic block [15].
The strengths and volumes of neurolytic agents shown below are not supported by scientific literature but are based only on clinical experience. Absolute alcohol is available in the United States as ethanol 98 % and phenol 100 %. For lower concentrations for clinical use, phenol must be diluted with saline by a compounding pharmacy.
Subarachnoid Neurolysis
Appropriate for well-localized, unilateral pain limited to a few dermatomes.
Cervical subarachnoid neurolysis should be performed at the spinal segment level to be blocked because cervical nerve roots pass horizontally from the cord through the intervertebral foramen [43].
Upper thoracic subarachnoid neurolysis should be performed at the vertebral interspace of the dermatome to be blocked. Middle and lower thoracic subarachnoid neurolysis should be performed one or two segments above the vertebral interspace of the dermatome to be blocked due to the anatomic course of the thoracic nerve roots.
Lumbar subarachnoid neurolysis should be performed at the T11–T12, and subarachnoid neurolysis for sacral dermatomes should be performed at the L1–L2 interspace.
Intrathecal neurolysis or rhizolysis of the dorsal root will provide a sensory block without a motor block.
Procedure
The patient should remain awake and alert throughout the procedure.
Sterile prep and drape.
Patient may be positioned sitting or lateral for initial needle positioning.
A 20- or 22-gauge 3.5-in. spinal needle is advanced to intrathecal space at level of desired dermatome.
After confirmation with (+) CSF, the patient is positioned for neurolytic injection.
Alcohol is hypobaric; position patient with painful side up; positioning is critical as alcohol diffuses quickly and sets up quickly. Patient should be positioned laterally with 45 % forward tilt. Bilateral blocks with alcohol may be achieved with the patient in the prone position.
Phenol is hyperbaric but diffuses out of glycerol slowly; therefore, positioning is less critical and may occur after injection. Patient should be positioned with painful side down with a 45 % posterior tilt.
Absolute alcohol or phenol 6 % in glycerin, up to 1 ml is injected.
Potential complications include painful setup (alcohol), coagulum with CSF (do not aspirate CSF prior to injection), bowel/bladder incontinence, lower extremity weakness, and motor block (Figs. 5.1 and 5.2).
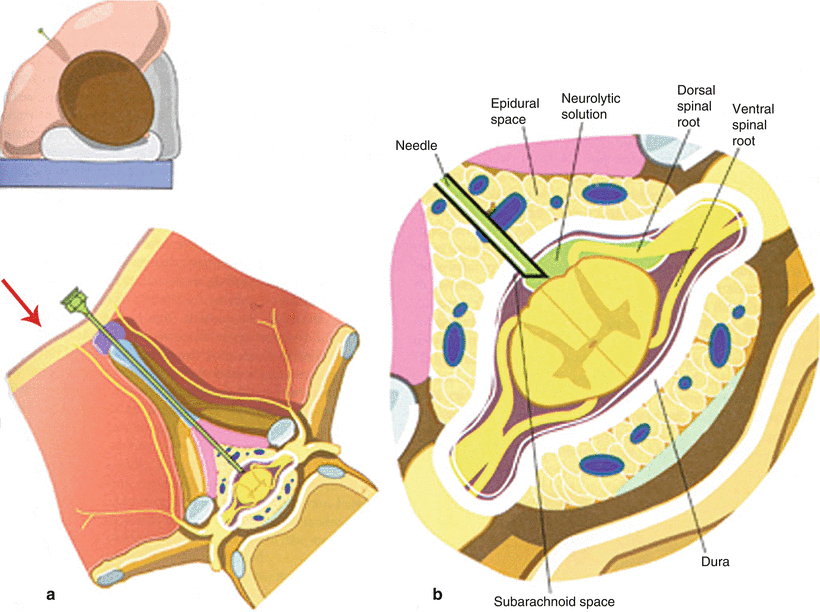
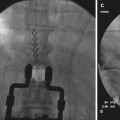
Fig. 5.1
Proper positioning of the patient with left-sided pain for intrathecal injection of alcohol (a) and close-up demonstration of proper needle entry into subarachnoid space (b). Note the 45° anterior tilt intended to bathe the posterior (sensory) nerve roots with hypobaric alcohol while sparing the anterior (motor) roots (With permissions from Waldman [44])
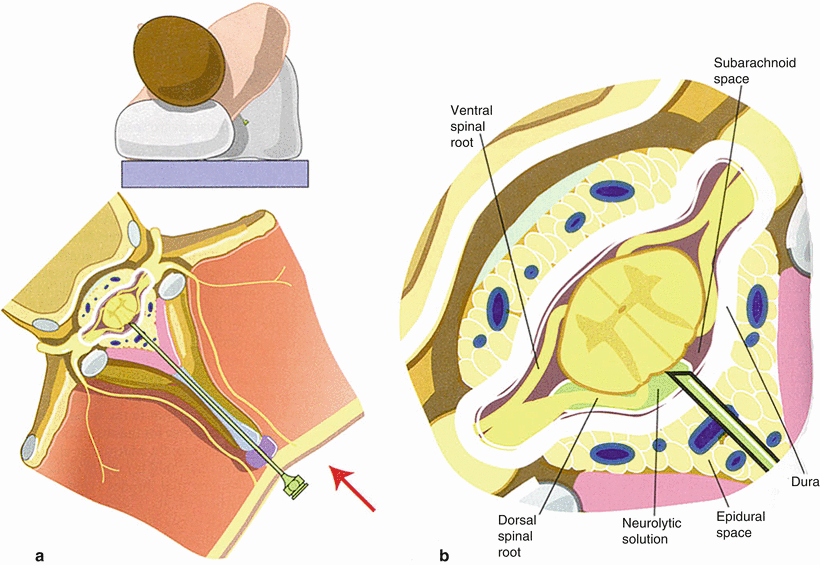
Fig. 5.2
Proper positioning of the patient with left-sided pain for intrathecal injection of phenol in glycerin (a) and close-up demonstration of proper need entry into the subarachnoid space (b). Note the 45° posterior tilt intended to bathe the posterior (sensory) nerve roots with hyperbaric phenol while sparing the anterior (motor) roots (From Waldman [45])
Epidural Neurolysis
Appropriate for well-localized bilateral pain limited to a few dermatomes including pain in the upper abdominal wall, thorax, or upper extremity. Positioning is less critical; thus, this approach may be an option when positioning for subarachnoid neurolysis is difficult. Epidural neurolysis has less predictable spread than with intrathecal neurolysis.
Procedure
The patient should remain awake and alert throughout the procedure.
The patient is positioned with painful side down in 45 % posterior tilt.
The patient is prepped and draped in a sterile manner.
Lidocaine 1 % local skin and subcutaneous tissue infiltration.
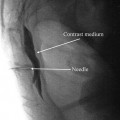
17–18-gauge Tuohy epidural needle inserted into epidural space with loss of resistance technique. Epidural catheter threaded under fluoroscopic guidance to level of painful dermatomes to be treated.
Injection of small volume of contrast is performed to confirm epidural spread and rule out intrathecal or intravascular spread.
Absolute alcohol 8–10 ml; phenol 15 % in glycerol/75 % of LA volume 8–10 ml is injected.
The patient should ideally remain in this position 40 min following injection.
Potential complications include motor block, numbness, neuritis, and deafferentation pain.
Peripheral Nerve Neurolysis
Appropriate for pain localized to a single peripheral sensory nerve. Most peripheral nerves are mixed; therefore, peripheral neurolysis carries a high risk of motor block as well [13].
Procedure: First, perform a prognostic block with local anesthetic in order to assess efficacy. The local anesthetic block will determine appropriateness of location of block as well as provide the patient with an opportunity to evaluate the effect with a short-term block. If the patient is uncomfortable with the numb sensation or motor weakness, a neurolytic block is not indicated.
Ultrasound should be considered for nerve localization during the procedure.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree