Endovenous thermal ablation for refluxing great saphenous veins (GSVs) has become increasingly common in practice over the past decades and has proven safe and effective, 1, 2, 3 replacing traditional ligation or stripping in several societal guidelines 4 as first-line therapy. Thermal ablative techniques include both laser and radiofrequency (RF) devices, previously discussed in detail in Chapter ▶ 6.
Thermal ablation methods require tumescent anesthesia, which provides several benefits, including compressive vein wall apposition to allow more efficient ablation, local anesthesia to avoid systemic sedation, and a heat sink to protect adjacent soft tissue from thermal injury. Nonetheless, despite its essential role for thermal ablation, administration of tumescent anesthesia adds to procedure time and has been shown to be more painful than the ablation procedure itself. 5 Additionally, there is also a small associated risk of lidocaine toxicity. 6 It should also be noted that postprocedural care for both laser and RF ablation includes the utilization of compression stockings, which itself can be a source of discomfort.
Though thermal ablation is highly effective and time-proven, there has been strong interest in the development of nonthermal ablative techniques as these obviate the need for tumescent anesthesia, since there is no heating of soft tissues, therefore potentially decreasing procedure time and procedure-associated pain. Nonthermal ablation also circumvents potential side effects intrinsic to thermal ablation including sensory nerve damage and bruising.
This chapter will detail the three nonthermal techniques with the greatest substance of science and literature behind them, including polidocanol (POL) endovenous microfoam sclerotherapy (Varithena), mechanochemical ablation (MOCA; ClariVein), and cyanoacrylate embolization (CAE; VenaSeal). Additional nonthermal techniques described at the end of the chapter are currently considered more experimental, with less published evidence.
10.2 Endovenous Microfoam Sclerotherapy/Varithena
10.2.1 Overview
Foam sclerotherapy refers to administration of a liquid sclerosant that has been mixed with gas to produce a foam preparation. While foam can be manually compounded via the Tessari technique (Chapter ▶ 7), Varithena is a pharmaceutical grade, low-density, injectable POL microfoam that is formulated via a proprietary canister system. Currently, in the United States, Varithena is the only Food and Drug Administration (FDA)–approved foam for treatment of incompetent GSVs. In the proprietary Varithena formulation, POL, the sclerosant agent, is mixed with oxygen, carbon dioxide, and an ultra-low amount of nitrogen within a canister to produce a 1% POL microfoam solution. 7, 8, 9, 10, 11, 12, 13, 14, 15
Absolute contraindications for foam sclerotherapy include severe allergy to the sclerosant, acute deep vein thrombosis (DVT) or pulmonary embolism, local infection in the limb to be treated, and long-lasting immobility. The procedure should be avoided in pregnant and nursing patients. Relative contraindications for foam sclerotherapy include severe peripheral arterial disease, high thromboembolic risk, and superficial thrombophlebitis. 16, 17
10.2.2 Mechanism of Action
The mechanism of action of sclerosant agents is discussed in detail in Chapter ▶ 7. In summary, the POL foam displaces blood instead of mixing with it, maximizing endothelial surface contact area and time. POL disrupts the osmotic barrier of the venous endothelium, leading to vessel wall damage and vasospasm. As a result, the interior surface of the vein becomes thrombogenic, leading to thrombosis and occlusion. The occluded vein is eventually replaced by fibrous connective tissue. 7, 8, 9, 10
As compared to manually compounded foam, the Varithena foam has been found to have smaller and more homogeneous bubble size ( ▶ Fig. 10.1). The median bubble diameter of Varithena is less than 100 μm and no bubbles are greater than 500 μm. Compared to physician-compounded foam, the Varithena foam has a slower degradation rate in ex-vivo models, which may correlate to increased vein contact time. 11

Fig. 10.1 Varithena foam (left) compared to manually compounded foam (right).
(Reproduced with permission from BTG.)
10.2.3 Varithena® Device and Technique
The Varithena® device holds two sterile, connected, 303-mL aluminum alloy canisters: one contains POL solution, 180 mg/18 mL (10 mg/mL), under a carbon dioxide atmosphere, and the second contains pressurized oxygen at approximately 5.4 bar absolute. The connector joins the two canisters allowing activation of the product ( ▶ Fig. 10.2). Once activated, Varithena injectable foam delivers a 1% POL solution, and each mL of Varithena injectable foam contains 1.3 mg of POL. One canister of Varithena generates a total volume of 90 mL of foam, which is sufficient to yield 45 mL of usable foam for intravenous injection. Once activated, the Varithena canister must be used within 7 days. 16

Fig. 10.2 Varithena canister with transfer unit attached.
(Reproduced with permission from BTG.)
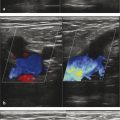
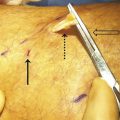
The vein to be treated is accessed via a micropuncture set or equivalent using aseptic technique under ultrasound guidance. The vein should be accessed distally. For example, when treating GSV insufficiency, the vessel is punctured near the level of the knee. The micropuncture sheath can be used for treatment or exchanged for an appropriately sized catheter (16–22 gauge). A manometer tubing (supplied with the Varithena kit) is prefilled with heparinized normal saline and then connected to the catheter. With the tube and catheter secure, the leg is elevated to 45 degrees. The Varithena canister is then activated to generate foam and then a transfer unit is attached to the canister ( ▶ Fig. 10.3). Up to 5 mL of foam at a time are transferred to a syringe. No visible air bubbles should be seen within the foam. Once extracted into the syringe, the foam must be administered within 75 seconds. 16
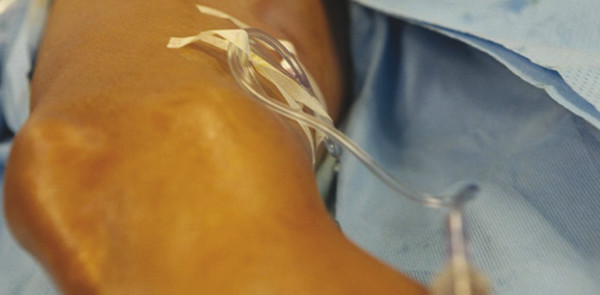
Fig. 10.3 Manometer tubing filled with Varithena foam, attached to intravenous catheter.
(Reproduced with permission from BTG.)
The syringe of freshly generated foam is connected to the manometer tubing. Foam is injected slowly under ultrasound monitoring and continued until the foam reaches a point 3 to 5 cm distal to the saphenofemoral junction (SFJ). Long catheters may also be used to precisely deposit foam throughout the vein. 18, 19 Additional foam may be injected distally to fill the distal GSV and major varicose tributaries. Venospasm of treated veins is confirmed via ultrasound, with some authors advocating manual compression on the SFJ during foam administration to minimize flow of foam into the femoral vein. 20 The recommended injection rate is approximately 1 mL/second in the GSV and 0.5 mL/second in accessory varicosities. A new sterile syringe is used after each injection. Up to 5 mL of foam may be used per injection and no more than 15 mL should be used per treatment session. Compression stockings with compression pads should be immediately applied and be worn for at least 2 weeks after treatment to assist with vein closure. 16
10.2.4 Outcomes
Literature suggests that manually compounded, nonproprietary foam sclerotherapy is less effective than thermal ablation of truncal incompetent veins. A meta-analysis evaluating 64 studies for anatomic success, defined as obliteration or disappearance of treated veins, demonstrated 3-year success rates of 95% for endovenous laser ablation, 84% for RF ablation, and 77% for foam sclerotherapy. 21
The VANISH-2 study 8 compared Varithena versus placebo in patients with SFJ incompetence due to reflux of the GSV or major accessory veins. Clinical improvement in symptoms of varicose veins at week 8 after treatment (as measured via a VVSymQ score) was seen in 80.5% of the pooled Varithena group (which included both 0.5 and 1.0% POL formulations) versus 21.2% of the placebo group. Duplex ultrasound response (defined as either elimination of SFJ reflux and/or complete occlusion of the incompetent vein seen at baseline) was seen in 85.6% of the pooled Varithena group versus 1.8% of the placebo group. 8
Results after treatment with Varithena were also found to be durable with ongoing improvement. Of the 56 patients followed to 1 year after treatment, 77.2% had improved symptoms at 8 weeks and 86.0% at 1 year. Similarly, 70.2% of patients reported improved appearance at 8 weeks and 87.7% at 1 year. 10
10.2.5 Complications
In Varithena placebo-controlled trials, 7, 8 the most common postprocedure adverse reaction with the Varithena was pain in the treated extremity (15%), along with limb discomfort (7–12%) and pain at the injection site (7–10%). A total of 80% of pain episodes in the treated limb resolved within 1 week. Additional adverse reactions included superficial thrombophlebitis (5–9%), nonocclusive thrombus extending from the superficial vein into the common femoral vein (2.9%), proximal DVT (1.7%), and distal DVT (1.1%). Contusion or hematoma of the injection site was seen in 9% of patients. There were no reported events of nerve damage. Hyperpigmentation of the treated limb was noted in 1.1% of patients treated with Varithena.
In the VANISH-2 trial 8 using Varithena foam, there were no clinically significant neurologic or visual adverse events. Cerebral gas embolism, however, has been found to be of concern when physician-compounding methods are used to create foams. The incidence of neurologic or visual adverse events with those preparations is approximately 2% and is predominantly transient. 11, 13 Complications related to foam sclerotherapy are discussed in detail in Chapters 7 and 9.
10.3 Mechanochemical Ablation/ClariVein®
10.3.1 Overview
MOCA refers to a hybrid method of endovenous ablation utilizing both mechanical abrasion via a rotating wire tip and simultaneous chemical ablation via injection of liquid sclerosant, either sodium tetradecyl sulfate (STS) or POL. The MOCA device ( ▶ Fig. 10.4) was developed in 2005 and is currently marketed under the tradename ClariVein®. Contraindications for MOCA are similar to those of sclerotherapy procedures discussed in Chapter ▶ 7 and include allergic reaction to the sclerosant agent, acute DVT or pulmonary embolism, local infection of the limb to be treated, peripheral arterial disease, and pregnancy. 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32
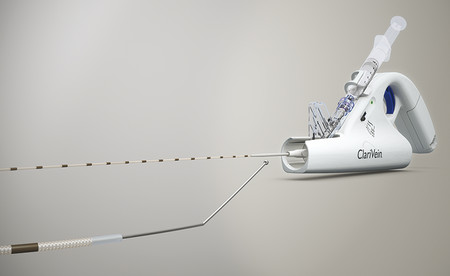
Fig. 10.4 The ClariVein device and wire tip.
(Reproduced with permission from Vascular Insights, LLC.)
10.3.2 Mechanism of Action
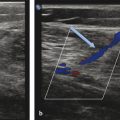
The MOCA technique combines mechanical damage to the endothelium caused by the rotating wire with the chemical damage caused by the infused sclerosant agent. The mechanical damage promotes coagulation activation by damaging the endothelium; induces local vasospasm and decreases the diameter of the treated vein; promotes better distribution of the sclerosant within the vascular lumen; and increases the action of the sclerosant agent by mechanical damage to the endothelium ( ▶ Fig. 10.5). The liquid sclerosant further damages the lipid cell membrane of the endothelium, ultimately resulting in occlusion and fibrosis of the treated vein. 22 Liquid rather than foam sclerosant is advised by the manufacturer for this hybrid technique due to lack of FDA approval for intravascular use of manually compounded foams as well as possible side effects and complications seen with foam administration. Histologic analysis of a GSV 1 year after MOCA demonstrated circumferential disappearance of the endothelium and fibrosis of the vein. The media was also considerably damaged with collagen changes. 22
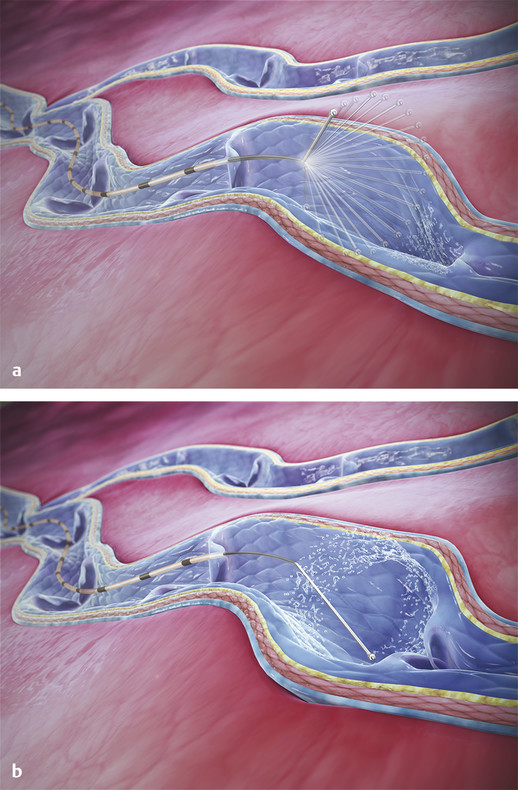
Fig. 10.5 (a) Rotating wire tip of the ClariVein device causes mechanical damage to the endothelium of the diseased vein. (b) Liquid sclerosant simultaneously released with wire rotation results in simultaneous chemical damage to the endothelium of the diseased vein.
(Reproduced with permission from Vascular Insights, LLC.)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree