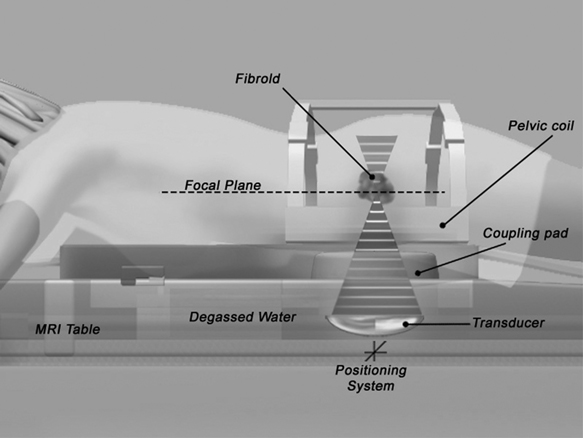
6 New Treatments for Uterine Fibroids Gary P. Siskin, Suzanne D. LeBlang, and Kristof Chwalisz For decades, there was very little innovation in the treatment of uterine fibroids.1 During that time, the standard treatment for fibroids had been hysterectomy or myomectomy, primarily because they are effective and offer a solution to the problem. There have been significant advances in surgical technique to avoid the morbidity associated with laparotomy,2 including laparoscopic and hysteroscopic resection of subserosal and submucosal fibroids, respectively. However, these approaches are often not appropriate for many patients and this has prompted the search for novel treatment options for these patients.1 With the success of uterine artery embolization (UAE) as an alternative to surgery, it now appears that a multitude of different approaches to treat these patients has been introduced into both the literature and into current medical practice. The purpose of this article is to review the different approaches to fibroid therapy and the data supporting their use. The fundamental concept behind lesion-based treatment options for uterine fibroids is that fibroids represent a focal problem within the uterus that can potentially be treated by directing therapy toward one lesion at a time. The most invasive form of this therapy is myomectomy because conceptually, this procedure involves removing specific lesions and leaving the remaining portions of the uterus intact. It is clear, however, that less invasive forms of management have become a popular alternative to traditional surgical resection. If a less invasive approach is desired, then nonsurgical lesion-based therapy will require accurate imaging guidance to be certain that therapy is being directed at abnormal tissue with the intention of sparing as much normal uterine tissue as possible. This concept is one that is familiar to most interventional radiologists who are involved with using ablation techniques (e.g., radiofrequency ablation [RFA], cryoablation, microwave ablation) to treat hepatic, renal, and pulmonary malignancies. In fact, many of these interventions have been applied in limited fashion toward the treatment of uterine fibroids and the clinical results will be reviewed in this section. A variety of thermal ablation techniques has been applied to the treatment of uterine fibroids. This includes RFA, cryoablation, laser ablation, and MR-guided focused ultrasound (MRgFUS). The use of RFA to achieve local control of a wide variety of tumors in many locations has become more accepted in recent years. It has been theorized that RFA treats fibroids by inducing coagulative necrosis and depressing the expression of estrogen and progesterone receptors.3 Bergamini et al4 were the first to report the use of laparoscopic RFA to treat symptomatic uterine fibroids. In this study, 18 patients, all of whom were older than age 40 and premenopausal with fewer than three fibroids were treated with RFA. All of the procedures were performed under general anesthesia using the system manufactured by Rita Medical Systems (part of Angiodynamics, Queens-bury, NY) under laparoscopic guidance. The operative time ranged from 20 to 40 minutes and there were no intraoperative or postoperative complications reported. Only two patients complained of mild abdominal pain, both of whom did not require postprocedure pain medication. All patients were observed overnight and discharged on the following day. Nine patients were available for 12-month imaging follow-up, which demonstrated a mean fibroid volume reduction of 85%. Significant improvements in both symptom severity and health-related quality of life were observed based on pre- and postprocedure administration of the Uterine Fibroids Symptom and Quality of Life (UFS-QOL) questionnaire. Ghezzi et al5 later reported the results of laparoscopic RFA on 25 patients with at least one-year follow-up, including the 18 patients reported by Bergamini et al.4 The median baseline diameter and volume of the dominant fibroid were 5.3 cm (range 3.0 to 8.6 cm) and 76.8 cm3 (range 14.8 to 332.8 cm3). In these patients, the median reduction in fibroid volume was 68.8% and 77.9% at 6 and 12 months, respectively. No intraoperative or postoperative complications were reported in this study. One patient required a hysterectomy for symptom recurrence at one year. All other patients had significant improvement in symptom severity and health-related quality of life. Milic et al6 have also reported their experience with laparoscopic RFA. They treated four patients with fibroids <6 cm in diameter with the LeVeen Needle Electrode system (Boston Scientific Corp., Natick, MA). These patients were observed for 6 hours and discharged home the same day. The procedure was technically successful in 3 of 4 patients: one patient had a firm, mobile, posterior fibroid that could not be treated. Symptomatic relief was achieved in two of the three treated patients: the one patient with persistent postprocedure pain was later found to have adenomyosis in addition to fibroids. Magnetic resonance imaging (MRI) performed at 3 months in the three treated patients, revealed lack of fibroid enhancement in all three patients. However, at 7 months, a focal area of enhancement within a treated fibroid was seen in one patient and this particular patient experienced recurrence of pain and bleeding within 9 months of the procedure. Kim et al7 also evaluated the use of percutaneous RFA for uterine fibroids, but did so in patients with large fibroids (>5 cm) who had been treated with UAE. Thirty-five patients were included in this study and were treated with moderate sedation. Significant improvements were seen in both symptom severity and health-related quality of life after the procedures were performed. In addition, the mean fibroid volume reduction was 56.5%. There were no immediate percutaneous RFA-related complications. However, one patient did have delayed drainage via the transabdominal tract, which was ultimately self-limiting. Kim et al7 theorized that percutaneous RFA may improve the clinical results of UAE by providing targeted treatments to areas of residual fibroid enhancement after UAE. Despite the small number of studies and the limited numbers of patients reported, the conclusions from the groups authoring the above articles are that RFA may offer a realistic alternative to surgery for symptomatic uterine fibroids. Laser myolysis was first described in 1990 by Donnez et al8 using hysteroscopic technique and by Nisolle et al9 in 1993 using laparoscopic technique. By placing laser fibers directly within the target fibroid, laser energy can be converted to heat. An attempt is made to coagulate the entire fibroid by placing laser fibers at multiple sites within the fibroid.10 As the temperature increases within the fibroid to ~56°C, protein denaturation develops, which causes coagulative necrosis within the fibroid.11 When initially reported in the mid-1990s, this laparoscopic procedure resulted in uterine volume decreases of 83% and fibroid volume decreases of 50 to 70% 3 to 6 months after the procedure.12,13 One problem associated with this technique was the significant incidence of pelvic adhesions in these patients. These adhesions appear to be secondary to the use of multiple fibroid punctures and thermal damage to the serosal surface.9 In 1999, Law et al reported on the use of MR-guided percutaneous laser ablation of uterine fibroids.14,15 The benefits of this approach were felt to be the real-time imaging guidance for needle placement and monitoring of the thermal ablation.16 The monitoring is important because a maximum ablation can be achieving before damaging the serosal and adjacent structures, which can potentially lead to adhesion formation.17 Law et al studied 12 patients who were awaiting hysterectomy and underwent laser myolysis. Technically, four MR-compatible 18 g needles were placed within the fibroid under MR guidance while the patient received intravenous (IV) sedation. Laser fibers were then advanced into the needles and the needles were withdrawn to expose the tip of the laser fiber. Laser light at a wavelength of 810 nm was then delivered to the fibroid tissue via each laser fiber. The mean ablation time was 15 minutes (range 10 to 25 minutes). Eleven of the 12 patients went home on the same day: one patient chose to remain in the hospital overnight. Of the 12 treated patients, 4 underwent hysterectomy with well-defined areas of coagulative necrosis seen within the excised fibroids. The remaining eight patients declined surgery after laser ablation and were found to have a fibroid volume reduction of 37.5% on MRI performed 3 months after the procedure. Law et al11 subsequently reported their experience with their first 30 patients. Treatment in 29 of 30 patients was considered technically successful; one patient terminated the procedure after 12 minutes due to abdominal pain. All but one patient was discharged on the day of the procedure. Of the 30 patients treated, 26 patients declined their planned surgery with all of these women reporting significant symptomatic improvement. After 3 months, the mean fibroid volume reduction was 37.5% (range 25 to 49%). As this pilot study continued, Hindley et al17 reported on 66 patients. MRI at 3 and 12 months revealed a fibroid volume reduction of 31% and 41%, respectively. All patients were noted to have a decrease in mean menstrual blood loss after treatment. In addition, the Menorrhagia Outcomes Questionnaire (MOQ), which was used to assess clinical outcomes, revealed that outcomes were not as good as those seen after hysterectomy in a historical control group, but that there were no significant differences in quality of life and satisfaction between the two groups of patients. Cryoablation appears to be the lesion-based approach that has the most literature supporting its use in patients with symptomatic uterine fibroids. It is thought that cooling the target tissue to -20°C with cryoablation destroys tissues by intracellular freezing and extracellular crystallization of interstitial water leading to cellular dehydration, thrombosis of small blood vessels, and mechanical damage to cellular integrity by expansion of large ice crystals within the interstitial space.18–22 Olive et al were the first to report the use of cryoablation to treat uterine fibroids.23 In this early report, cryoablation was performed using laparoscopic guidance on 14 patients; the authors subsequently reported a 10% fibroid volume reduction on follow-up imaging. Since then, other studies have been performed evaluating the use of laparoscopic cryoablation. Ciavattini et al25 treated 76 fibroids in 61 patients under general anesthesia with procedure times ranging from 20 to 60 minutes.24–28 After the procedure, 8.3% of patients complained of pain and 5% had a postoperative fever. Most patients (83.6%) reported symptomatic relief after the procedure and the mean fibroid volume reduction was 60.3% and 69.8% at 12 and 24 months, respectively. Zupi et al26 subsequently treated 20 patients using this technique. All procedures were performed with general anesthesia and all patients were discharged within 24 hours. No postprocedure analgesia was required and no procedural complications were reported. During follow-up, 95% of patients reported elimination or improvement of their symptoms; the mean fibroid volume reduction was 24.9% and 60% at 1 and 12 months, respectively.27 The ultrasound findings were further evaluated in 10 of these patients by Exacous-tos et al, who found decreased blood flow in all treated fibroids, even when peripheral surrounding vessels remained intact.28 This was seen 1 month after treatment and remained unchanged 3 to 6 months after treatment. In addition, a hyperechoic avascular central zone was seen in 80% of treated fibroids that persisted (although reduced in size) 6 months after treatment. There is limited data on pregnancy outcomes after laparoscopic cryomyolysis. Ciavattini et al29 reported their experience with nine pregnancies in nine patients who underwent this procedure to treat symptomatic uterine fibroids. Two of these patients had an early miscarriage while seven pregnant patients had a regular, uncomplicated course with three patients delivering by cesarean section and four patients delivering vaginally. Therefore, it was concluded that this procedure does not compromise a good pregnancy outcome and vaginal delivery. In 2001, reports regarding the percutaneous performance of cryoablation using MRI guidance became available. The first report was by Sewell et al30 who treated two patients with large symptomatic fibroids. A 10F access sheath was placed transabdominally into the target fibroid and a 6F cryoablation probe was then advanced through the sheath into the fibroid. Cryoablation was performed until the “ice ball,” which was well seen on MRI, encompassed the fibroid with minimal overlap into the surrounding myometrium. Once the cryoprobe was removed, the access sheath was filled with SurgiSeal (Confluent Surgical, Inc., Waltham, MA) for hemostasis. After 2 and 3 months, MRI revealed fibroid volume reductions of 65% and 53%, respectively. Cowan et al31 later reported a similar mean fibroid volume reduction of 65% when nine patients were treated using a similar technique. In this series, complications were reported including nausea requiring an overnight admission, mild foot drop due to a peroneal nerve defect that resolved after 4 months, and laceration of a vessel coursing over the serosal surface of a fibroid that led to bleeding requiring laparotomy and myomectomy. Sakuhara et al32 also reported on their experience with six patients undergoing MR-guided cryoablation. All of these patients experienced a fever after treatment with one patient requiring surgical drainage of an abscess in the probe channel. The mean fibroid volume reduction rate in these patients in these patients was 79.4% at 9 to 12 months. Dohi et al were the first to report the use of cryoablation from a transvaginal approach to treat eight patients with symptomatic fibroids.18 Cryoprobes were introduced utilizing MRI guidance under epidural and local anesthesia into the targeted fibroids using a transvaginal approach. Lower abdominal pain occurred after the procedure in 3 patients, which was addressed with analgesic medication. All patients were discharged on the day following the procedure. Clinically, the menstrual score improved in 75% of the patients with pain and anemia successfully addressed in all patients with these symptoms. At 6 to 7 weeks after the procedure, the mean fibroid volume reduction was 59%; at 9 to 12 months, it was 67.7% (in five patients). Interestingly, Dohi et al suggested that the optimal size of the ice ball formed during cryoablation may not need to be larger than the fibroid to cause alleviation of symptoms in these patients. When evaluating the data for lesion-based therapy (irrespective of the actual technique used) and the imaging modality used for guidance (laparoscopy or MRI), it appears that the short-term data are promising in terms of the ability of these procedures to improve symptoms and reduce fibroid volume. An additional benefit of these procedures is the clear absence of significant postprocedure pain, a finding that differentiates these procedures from UAE and other more invasive therapies. This may be due to the fact that these targeted therapies are successful in limiting treatment to the fibroids, reducing the effect of treatment on normal myometrium. This may therefore represent an advantage for this type of therapy when compared with a more organ-based treatment such as UAE. However, a lesion-based approach also has limitations. Because each fibroid requires individually targeted therapy, there are restrictions to the number of fibroids that can be present before this would be considered an unreasonable approach. If multiple procedures are required to treat multiple fibroids, then the overall risk of utilizing this approach would be increased as well. In addition, if small fibroids are left untreated, then symptom recurrence may become a potential long-term issue. Multiple fibroids were therefore a limitation that was mentioned in many of the above-quoted studies and was often used as selection criteria for deciding which patients would be eligible for participation. Given the fact that many patients present with multiple fibroids, these ablative techniques may not be appropriate for many of the patients who present with symptomatic fibroids. An additional limitation is that at this time, all of the patients described above were not evaluated with contrast-enhanced MRI. The global experience with UAE has emphasized the importance of utilizing contrast-enhanced MRI as the imaging modality of choice after UAE to confirm that fibroid infarction has been induced by the procedure.33 Without this information, it is not possible to know if these targeted ablation techniques result in tissue infarction. Although this may open up the possibility of future recurrence, it also may help explain why these patients experienced less postprocedure pain than typically seen after UAE. Therefore, additional studies with contrast-enhanced MRI as the follow-up imaging modality would be required to better characterize the tissue changes induced by these procedures. The use of MR-guided focused ultrasound surgery (MRg-FUS) to treat patients with symptomatic uterine fibroids is an exciting step forward in lesion-based therapy because it truly defines noninvasive medicine. Although MRgFUS has been studied for over six decades,34,35 it was only approved by the United States Food and Drug Administration (FDA) for the treatment of uterine fibroids in October, 2004. By definition, noninvasive surgery means that the skin remains intact with no incisions, catheters, or laparoscopic portholes. Despite the fact that this technology has been evaluated for a long time, improvements in MRI, MR thermometry images, and the ability to combine the software components of the MRI machine with the focused ultrasound transducer has propelled it into clinical use. This procedure delivers high-energy ultrasound waves into the fibroid under direct MRI guidance. The patient lies in the prone position with the pelvis overlying the phased array ultrasound transducer, which is encased in a degassed water tank within the MRI table (Fig. 6.1). This table is able to dock with a 1.5 or 3.0 Tesla MRI scanner. Similar to how a magnifying glass focuses light energy, the ultrasound waves are focused into a small region of tissue inside the fibroid called the focal spot;35 this can range in size from a small jellybean to a large coffee bean. The MRI T2-weighted planning images in three planes are utilized to determine the focal spot location. The ultrasound beam path is depicted overlying these anatomic images to ensure a safe passageway into the fibroid. The transducer can be tilted ~15 to 20 degrees in all directions to avoid scars or nearby loops of bowel. During the 20- to 30-second sonications when the ultrasound waves are focused into the fibroid, heat is generated in the focal spot resulting in thermal ablation. Studies have shown that if the temperature remains above 57°C for >1 second, then coagulative necrosis occurs leading to irreversible cell death.36,37 Every 3 seconds during the sonications, MR thermometry images are obtained that follow the temperature within the focal spot during the sonciation to ensure that the temperature is high enough to induce cell death and tissue ablation. Various parameters can be altered during the procedure to reach critical temperatures such as the amount of energy in the ultrasound beam, the frequency of the ultrasound beam, the size of the focal spot, and the duration of the sonications. Fig. 6.1 Diagrammatic representation of a patient in the prone position receiving magnetic resonance-guided focused ultrasound (MRgFUS) to treat a uterine fibroid. Patient selection is important to ensure a safe and effective outcome after MRgFUS. The published clinical trials limited inclusion to premenopausal women with no plans for future childbearing with a uterus <24 weeks in size. The timing of the procedure relative to the phase of the menstrual cycle has not been shown to be important.38 A screening MRI of the pelvis with and without contrast is necessary prior to the procedure to determine candidacy and several parameters should be noted carefully. Perhaps the most important reason to screen these patients is to ensure that the patient does have fibroids and not other pathology that can mimic a fibroid on the ultrasound images (e.g., endometrial cancer, sarcoma, or a focal adeno-myoma). Patients with more than five fibroids are usually not good candidates because the time to ablate these fibroids can be prohibitively long. In addition, the signal characteristics of the fibroid should be assessed because low-signal lesions on T2-weighted images tend to respond better to MRgFUS than high-signal lesions.39 Postcontrast images should demonstrate diffuse enhancement of the fibroid ensuring that it is not already necrotic or hemorrhagic as there would be no viable tissue to ablate in such cases. Scrutiny of the images for bowel loops interposed between the anterior abdominal wall and the uterus is critical to note as the air inside these bowel loops could deflect the ultrasound beam and heat other surrounding tissues. Abdominal scar tissue, including that seen after abdominal liposuction, represents a contraindication to this procedure as well, if the scar tissue would be in the path of the ultrasound beam. In our practice, 73% of patients screened with MRI are found to be good candidates for MRgFUS (LeBlang). Findings that have excluded patients include malignancy (including endometrial cancer and uterine leiomyosarcoma), adenomyosis, nonenhancing fibroids, large number of fibroids, endometrial polyps, fibroids of inappropriate size (too large and too small), highly vascularized fibroids, clips along the beam path, or fibroids that are inaccessible to the beam path. Prior to the procedure, patients are instructed to shave the area from the umbilicus to the pubic symphysis because hair increases the risk of thermal injury to the skin.40 No oils or creams can be placed on the skin of the lower abdominal wall before treatment. A Foley catheter is placed to keep the bladder empty because a distending bladder can cause changes in the uterine position compared with the T2-weighted planning images, resulting in misplacement of the focal spot.40 The Foley catheter is also needed to maintain the bladder filled at a constant level when used to push intervening bowel loops out of the beam path. These bowel loops can be displaced laterally and superiorly by filling the bladder immediately prior to the procedure. Conscious sedation is typically used to minimize patient motion and minimize any discomfort during the procedure. Once this technology was shown to effectively reduce uterine fibroid tumor size in a nude mouse model,41 trials began in humans. The initial feasibility data obtained on patients with symptomatic uterine fibroids was reported by Tempany et al.42
Lesion-based Treatment
Thermal Ablation
Magnetic Resonance–guided Focused Ultrasound Surgery
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree