Fig. 19.1
Ligaments and peritoneal spaces in upper abdomen. A – lesser omentum, B – greater peritoneal cavity, C – gastrosplenic ligament, D – lesser sac, E – splenorenal ligament, K – kidney, L – liver, S – spleen, St – stomach
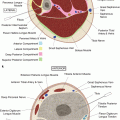
The retroperitoneum lies between the parietal peritoneum anteriorly and the transversalis fascia posteriorly. It is divided into three compartments by well-defined fascial planes. The perirenal space is enclosed by Gerota’s fascia. It extends across the midline, abuts the bare area of the liver on the right, and the subphrenic space on the left. It communicates with the mediastinum via the diaphragmatic hiatus. The posterior pararenal space is open toward the pelvis inferiorly but bound superiorly by fusion of the quadratus lumborum fascia and the posterior perirenal fascia. The anterior pararenal space communicates across the midline, superiorly it extends to the dome of the diaphragm and mediastinum, inferiorly it communicates with the pelvis, and below the inferior renal cone it communicates with the posterior pararenal space (Fig. 19.2) [2].
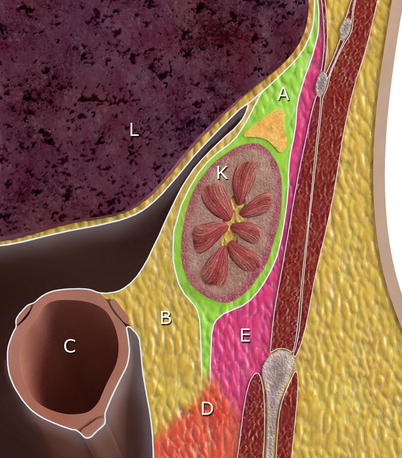

Fig. 19.2
Retroperitoneal compartments. A – perirenal space contains the kidney, the adrenal gland, the proximal ureter, and fat; B – anterior pararenal space contains the pancreas, duodenum, ascending and descending colon, and fat; C – transverse colon; D – infrarenal retroperitoneal space; E – posterior pararenal space contains fat, lymph nodes, nerves, and blood vessels; K – kidney; L – liver
Pathways for Spread of Disease in the Abdominal Cavity
An understanding of the possible mechanisms by which disease can spread within the abdominal cavity can greatly enhance the radiologist’s ability to identify the epicenter of a disease process and predict the likely path of disease progression. Pathways for disease transmission within the abdominal cavity include hematogenous, transperitoneal, and subperitoneal spread [3]. Transperitoneal dissemination is confined to the peritoneal cavity and includes ascites, abscesses, pneumoperitoneum, and peritoneal tumor deposits. Subperitoneal spread of disease refers to disease spread within the ligaments, mesentery, mesocolon, or under the peritoneal surface of the organs [4]. This interconnecting potential space represents a significant conduit for bidirectional spread of disease within and between the peritoneal and retroperitoneal compartments. Subperitoneal spread may also follow the lymphatic drainage of organs along the blood vessels in the mesentery, mesocolon, and ligaments.
Pathology
Retroperitoneal and mesenteric lymphadenopathy can be an unexpected imaging finding and represent the first sign of disease. Given the increased frequency with which lymph nodes are identified on cross-sectional imaging and the wide differential diagnosis for lymphadenopathy in these regions, a careful evaluation of lymph node imaging characteristics (size, distribution, CT attenuation coefficient, signal changes on MRI, post-contrast enhancement) should be performed. Correlation with the patient’s clinical history should be made in an effort to rule out a neoplastic condition. Bulky retroperitoneal lymphadenopathy in association with mesenteric lymphadenopathy is suggestive of lymphoma. Abdominal lymphadenopathy, disproportionately involving the mesentery, is suggestive of tuberculosis [5].
The most common soft tissue masses to affect the retroperitoneum and peritoneal structures are metastases and lymphoma [6]. Metastases to the retroperitoneum most commonly originate in the pelvis (rectum, cervix, prostate) [7]. Peritoneal carcinomatosis and metastases to omentum and mesentery frequently arise from tumors of the ovary, colon, stomach, pancreas, uterus, and bladder [8–11]. Tuberculosis can closely mimic peritoneal carcinomatosis radiologically. Forty to eighty percentage of gastrointestinal carcinoid tumors metastasize directly or via lymphatic spread to the mesentery [12, 13]. The infiltrative pattern of mesenteric disease seen with carcinoid, desmoid tumors, retractile mesenteritis, and peritoneal mesothelioma can sometimes be difficult to differentiate on CT.
Primary neoplasms of the retroperitoneum are uncommon and are derived from mesenchyme, neurogenic tissue, or embryonic rests. Primary malignant tumors are about four times more common than benign neoplasms [14]. Liposarcoma and leiomyosarcoma account for over 90 % of malignant tumors. Approximately two-thirds of omental tumors are benign and include leiomyomas, lipomas, and neurofibromas. Malignant tumors include leiomyosarcoma, liposarcoma, fibrosarcoma, mesothelioma, and hemangiopericytoma [6, 15]. Primary peritoneal tumors are a rare group of tumors that arise from the mesothelial and submesothelial layers of the peritoneum [16]. They include malignant mesothelioma, multicystic mesothelioma, primary peritoneal serous carcinoma, leiomyomatosis peritonealis disseminata, and desmoplastic small round cell tumor. Many of these tumors have imaging findings similar to peritoneal carcinomatosis.
Indications
For many patients, the information provided by the clinical history and imaging studies is insufficient to exclude a malignancy. The imaging features of many primary neoplasms of the retroperitoneum and peritoneum are nonspecific. While the presence of calcifications, fat, necrosis, or cystic components are key to providing a differential diagnosis, there is often considerable overlap between neoplastic and nonneoplastic lesions and between benign and malignant tumors. Moreover, cytological analysis of ascitic fluid rarely results in site-specific tumor diagnoses in cases of peritoneal carcinomatosis [17]. Percutaneous needle biopsy of the retroperitoneum, mesentery, or omentum may be required to establish the benign or malignant nature of a lesion, to obtain material for microbiologic analysis in patients with known or suspected infections, and to stage patients with known or suspected malignancy when local spread or distant metastasis is suspected. A repeat biopsy may be required if there is a strong clinical suspicion of malignancy and the previous biopsy result was negative or inconclusive, or the result is discordant with the clinical and imaging findings.
At our institution, one of the most common indications for percutaneous biopsy of a retroperitoneal or mesenteric soft tissue mass is for the diagnosis, staging, and restaging of lymphoma. While the clinical practice guidelines issued by the European Society of Medical Oncology recommend excision biopsy for the diagnosis of newly diagnosed and relapsed lymphoma (except in emergency circumstances) [18], numerous studies support the use of core biopsy in providing a definitive histological diagnosis. Core biopsy diagnostic rates of 83–88 % for lymphoma have been quoted for nodes inside and outside of the retroperitoneum [19–22]. Controversy also surrounds the diagnosis of retroperitoneal soft tissue sarcomas. In the past, definitive diagnosis and treatment of these tumors was achieved with surgical resection. High local recurrence rates of 50–60 % at 5 years and 90 % at 10 years have led to efforts to downstage the disease with preoperative and intraoperative radiotherapy [23–26]. In this patient, population pretreatment percutaneous biopsy is appropriate. While surgical excision is the treatment of choice for many cystic masses in the retroperitoneum and mesentery, percutaneous needle aspiration can be useful to confirm the nature of a nonneoplastic cystic mass, e.g., urinoma, lymphocele, hematoma, or pseudocyst.
The radiologist’s pre-procedure evaluation should include a review of the patient’s clinical history and physical examination and correlation with available imaging studies. With a differential diagnosis in hand, the radiologist must determine if a diagnosis is possible with the evidence available, whether the diagnosis can be confirmed by less invasive means or that biopsy is indeed indicated. Not all lesions identified by imaging need to be biopsied, (e.g., FDG-avid mesenteric lymph nodes on PET-CT may be sufficient to confirm the presence of metastatic disease; an enhancing soft tissue mass involving bowel, with linear areas of soft tissue attenuation radiating outward in the mesenteric fat, is a CT finding strongly suggestive of carcinoid tumor). In order to avoid unnecessary and possibly hazardous interventions, the radiologist must be familiar with common “pseudotumors” and normal variants. Left-sided paraortic lymphadenopthy can mimic duplication of or left inferior vena cava (IVC). Retrocrural lymphadenopathy may mimic an enlarged azygos vein in the retrocrural space. Retroperitoneal lymphadenopathy can mimic circumaortic left renal vein. Retroperitoneal fibrosis is an inflammatory disorder that may be misinterpreted as a malignant process, as it envelops the aorta and IVC, often displacing and encasing the ureters. Other nonneoplastic entities that can have a mass-like appearance on CT include cystic masses (hematoma, urinoma, lymphocele, pseudocyst, duplication cyst), omental torsion, omental infarction, and inflammatory pseudotumor.
Patient Selection and Pre-procedure Planning
Before embarking upon biopsy of a deep retroperitoneal or mesenteric lesion, consideration should be given to biopsy of more accessible sites, e.g., the axillary or inguinal regions in the case of lymphadenopathy (Fig. 19.3). To minimize the risks associated with biopsy of deep lesions, the safest and shortest needle trajectory should be identified. A careful risk-benefit assessment should be performed prior to trans-intestinal biopsy of deep mesenteric lesions or trans-caval biopsy of retroperitoneal lesions. Biopsy may be contraindicated if the lesion is highly vascular. Markedly hypervascular tumors include paragangliomas and hemangiopericytomas. Moderately hypervascular tumors include some of the soft tissue sarcomas including leiomyosarcoma which can also be partly intravascular. Hypervascular retroperitoneal lymphadenopathy is a feature of Kaposi’s sarcoma and Castleman disease.
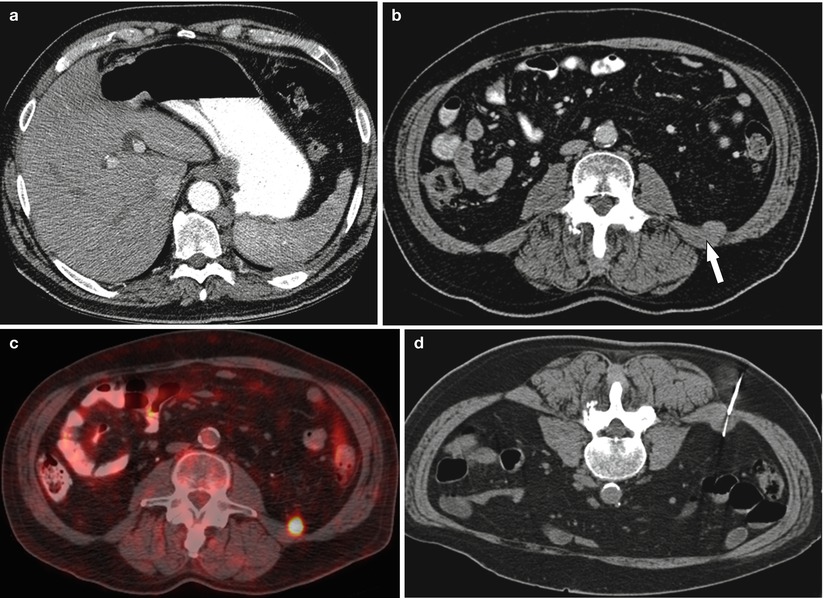

Fig. 19.3
A 66-year-old male with lymphadenopathy worrisome for lymphoproliferative disorder. (a) Contrast-enhanced CT abdomen axial image shows retrocrural lymph node. (b, c) Review of the PET CT revealed a more superficial and metabolically active lesion (arrow) contiguous with the quadrates lumborum fascia in the posterior pararenal space. (d) CT-guided biopsy was performed of this alternative site using a posterior approach. Pathology revealed a large B-cell lymphoma
Many retroperitoneal and deep mesenteric biopsies are performed with the patient. Close proximity to viscera, e.g., kidneys or bowel, may require breath holding during the procedure. The patient’s ability to cooperate and lie supine or prone should be assessed. Patient comfort and cooperation may be optimized with the use of moderate sedation administered by dedicated nursing staff. At our institution, respiratory compromise, decompensated cardiac failure, morbid obesity and diagnosed sleep apnea, pregnancy, pediatric patient, and inability to lie in the required position for the procedure are among some of the reasons that referral to anesthesia may be required.
A history of anticoagulation therapy and antiplatelet agents should be obtained. The Society of Interventional Radiology provides guidelines for the cessation of these drugs in patients undergoing percutaneous biopsy [27]. At our institution, aspirin and clopidogrel are held 5 days and coumadin 3–5 days prior to percutaneous biopsy. Coagulation status is tested in those on coumadin therapy and those with known or suspected liver disease. If the risks associated with complete cessation of anticoagulation are high, the patient is converted to unfractionated heparin (biological half-life 1–2 h). Patients on low molecular weight heparin have one dose held prior to the procedure. In patients with malignancy, biopsy of mesenteric and retroperitoneal lesions may involve additional risks owing to treatment-related coagulation disorders or the cancer itself, e.g., chemotherapy-induced thrombocytopenia. Although numerous studies have demonstrated a relatively low predictive value of abnormal laboratory screening parameters in predicting bleeding, laboratory criteria for biopsy at our institution include a platelet count greater than 50 × 109 and an international normalized ratio of less than 1.6 within 1 month before the procedure.
Contraindications [27]
There are no absolute contraindications for percutaneous needle biopsy. Relative contraindications may include:
1.
Significant coagulopathy that cannot be adequately corrected
2.
Severely compromised cardiopulmonary function or hemodynamic instability
3.
Lack of a safe pathway to the lesion
4.
Inability of the patient to cooperate with, or to be positioned for, the procedure
5.
Pregnancy in cases when imaging guidance involves ionizing radiation
Imaging Modality
The choice of imaging modality is based on equipment availability, radiologist preference, the size and location of the target lesion, patient body habitus, potential access routes, the ability to visualize the lesion, and cost. Factors to be considered in a patient with a high body mass index include the maximum weight load of the CT/MRI table, the ability to visualize the lesion on ultrasonography, and the ability of the interventional radiologist to compress the subcutaneous tissue with the ultrasound probe while performing the biopsy. Although many interventional radiologists prefer to perform CT-guided biopsies of retroperitoneal and mesenteric lesions in obese patients, ultrasound-guided biopsy has been demonstrated to be very effective in this subset of patients [28].
CT
CT is traditionally used as the modality for image-guided biopsy of retroperitoneal and deep mesenteric lesions [29, 30]. CT provides high spatial and contrast resolution and excellent delineation of intervening structures, such as vessels and bowel, thus enabling accurate needle localization (Fig. 19.4). The operator can easily correlate the findings on CT and functional imaging (e.g., positron emission tomography (PET)) to target the appropriate part of the mass for biopsy and to avoid areas of necrosis (Fig. 19.5). Performing CT-guided biopsies is associated with a shorter learning curve than performing ultrasound-guided biopsies, increasing the reliability of CT-guided biopsy among the general population of radiologists. Standard CT acquisition parameters for mesenteric/retroperitoneal biopsies are 3–5-mm-thick contiguous transverse sections.
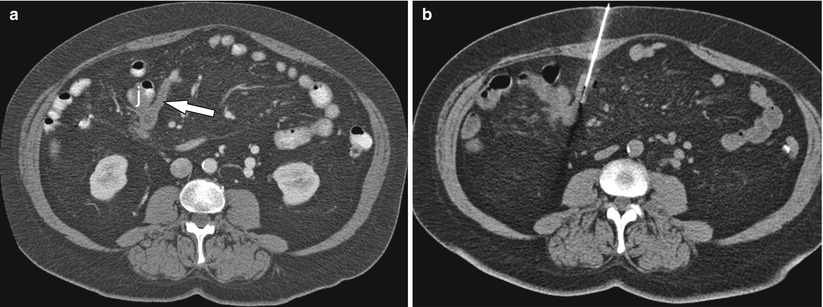
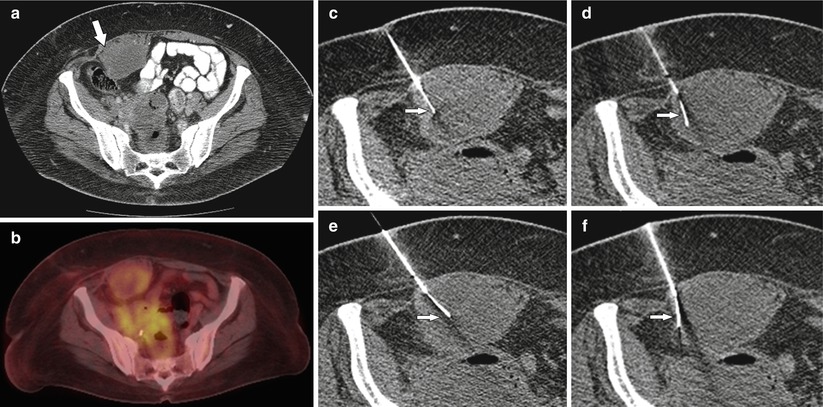

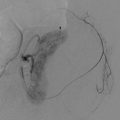
Fig. 19.4
A 65-year-old female status post right hemicolectomy for colonic carcinoma. (a) Contrast-enhanced CT abdomen axial image demonstrated a mass (arrow) involving the proximal small bowel mesentery and adjacent loop of jejunum (j). (b) CT-guided tissue sampling demonstrated metastatic colorectal carcinoma

Fig. 19.5
A 62-year-old male with a history of sigmoid carcinoma treated with sigmoid colectomy and adjuvant chemotherapy. (a) Contrast-enhanced CT abdomen axial image demonstrated a partly necrotic mass involving the right rectus abdominis muscle (arrow). (b) PET CT shows greater metabolic activity the periphery of the mass. (c) CT-guided biopsy using coaxial technique was performed. (d–f) By curving the tip of the core biopsy needle (arrows), it was possible to acquire sufficient tissue for a diagnosis of metastatic mucin-producing adenocarcinoma
CT fluoroscopy combines the advantages of the high-resolution imaging of CT with the real-time imaging of fluoroscopy. CT fluoroscopy can be especially useful for needle placement in omental or mesenteric lesions that move when the patient breathes or that may be intermittently surrounded by bowel loops [31, 32]. When using this modality, the operator should be aware of the available techniques to reduce radiation exposure for both the operator and the patient, including dedicated needle holders to keep the operator’s hand away from the gantry, using a low tube potential and low tube current, and using CT fluoroscopy intermittently during needle advancement instead of continuously [33–37].
Ultrasonography
In many countries in Europe and Asia, ultrasonography is the preferred guidance technique for biopsy in many regions of the body, including the mesentery (Fig. 19.6) [28, 38–41]. Advantages of ultrasonography include real-time imaging capabilities, the ability of color Doppler to delineate major vascular structures in and around the target lesion, the capacity to identify alternative access routes to the target lesion by angling the probe away from the axial plane, lack of exposure to ionizing radiation, decreased procedure time, and lower cost. Application of transducer pressure can displace and compress overlying fat or bowel loops, thus reducing the needle-path distance. In experienced hands, ultrasound-guided biopsy has been demonstrated to be as effective as CT-guided biopsy in establishing site-specific diagnosis in patients with peritoneal carcinomatosis [42].
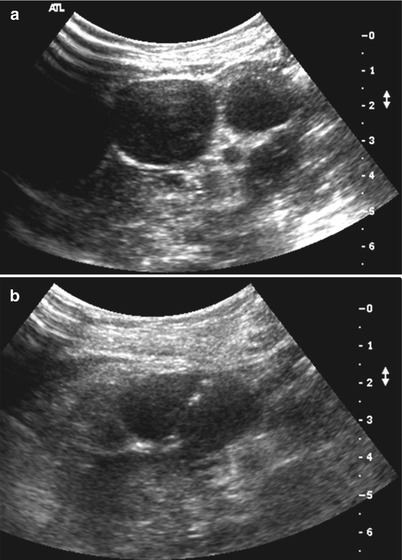

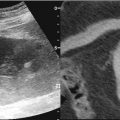
Fig. 19.6
A 31-year-old female presented with abdominal distension. (a) Ultrasound abdomen showed multiple hypoechoic mesenteric masses worrisome for lymphoma. (b) Ultrasound-guided biopsy was performed of a more superficial lesion located beneath the anterior abdominal wall. Pathology confirmed a diagnosis of B-cell lymphoma
The major drawback of ultrasound-guided biopsy is the fact that it is an operator-dependent method and thus may have low reproducibility. For those less experienced in ultrasound-guided interventions, a needle guide can facilitate visualization of the needle, reduce the time spent searching for the needle tip, and enable biopsy to be performed during a single breath-hold. This technique also ensures that the sampling is limited to the lesion.
Other drawbacks include the poor visualization of small or deep lesions and lesions overlying bowel or bone [43, 44]. While deep mesenteric and retroperitoneal lesions are often accessed via CT guidance, some authors have reported that ultrasonography is an accurate and safe guidance technique in the biopsy of small or deep lesions or lesions obscured by overlying bowel or bone [28, 45]. A number of new innovative ultrasound tools are now available that are likely to increase radiologist confidence in interventional studies. Fusion of ultrasound images with previously acquired imaging modalities like CT and MR combines the advantages of real-time ultrasound imaging with the high spatial and contrast resolution of CT, MR, or PET. The development of a “GPS-like technology” enables the radiologist to track and mark a patient’s anatomy during the ultrasound exam and in particular to track “hot spots” found on previous CT, MRI, or PET CT studies. These tools may be particularly beneficial for guiding biopsy of deep-seated lymph nodes and small soft tissue masses. Ultrasound-guided biopsy of deep lesions is usually performed with a convex probe. High-frequency linear probes are reserved for thin patients or superficial peritoneal lesions.
MRI
The superb soft tissue contrast resolution achieved with MRI makes it a useful diagnostic tool for localizing and staging retroperitoneal and mesenteric soft tissue masses. MRI guidance of percutaneous biopsies is becoming more popular with the advent of new open-configuration MRI systems, continuing development in the field of MRI-compatible instruments, and ultrafast MRI sequences that allow real-time imaging [46, 47]. Other advantages of MRI include its multiplanar capabilities, the lack of ionizing radiation, and its ability to visualize vessels without a contrast agent. Multiplanar imaging allows the radiologist to identify the safest trajectory for biopsy and maintain continuous visualization of the needle trajectory in that plane throughout the procedure. This is particularly relevant for posterior paraspinal and anterior approaches to deep retroperitoneal and mesenteric lesions that lie in close proximity to vessels and bowel loops. The higher cost associated with MR-guided interventions is largely attributed to the cost of MR-compatible instruments. With increased use, it is likely that the cost differential will diminish. These characteristics, in addition to a low complication rate and a high rate of adequate specimens, make MRI-guided biopsy a reasonable alternative to CT-guided biopsy [48].
Techniques
Retroperitoneal and mesenteric lesions can be accessed from an anterior or posterior approach. When the posterior approach is performed, the needle usually passes alongside or through the quadratus lumborum and psoas muscles (Fig. 19.7). Although this is a safe trajectory, the operator should be aware that passing the needle through these muscles decreases the possibility of correct needle trajectory. Some proponents of a posterior paraspinal approach argue that in the event of a bleeding complication, the result would be a contained retroperitoneal hematoma rather than free intraperitoneal hemorrhage [49].
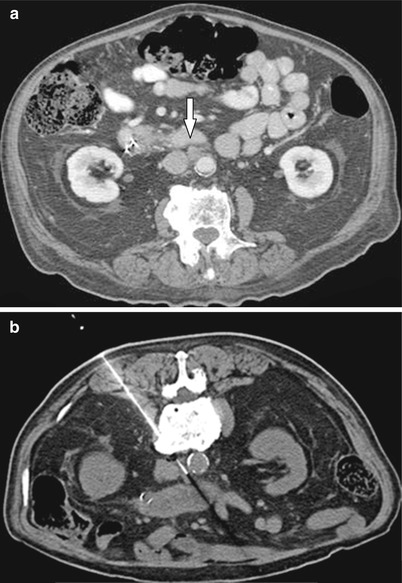

Fig. 19.7




A 78-year-old male undergoing chemotherapy for pancreatic carcinoma. (a) Contrast-enhanced CT axial image demonstrates aortocaval lymph node (open arrow) suspicious for malignancy. (b) CT-guided biopsy using a posterior approach was performed avoiding the aorta (a) and the IVC (ivc). Pathology confirmed metastatic adenocarcinoma
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree