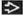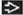Nonspecific Interstitial Pneumonia
Jud W. Gurney, MD, FACR
Tan-Lucien H. Mohammed, MD, FCCP
Key Facts
Terminology
1 of the idiopathic interstitial pneumonias, less common than idiopathic pulmonary fibrosis (IPF), but with better prognosis than IPF
Imaging Findings
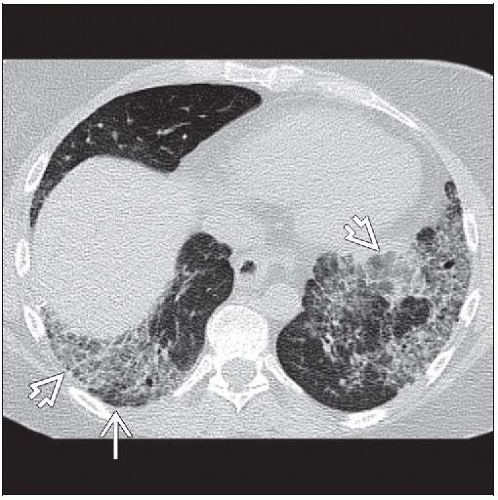
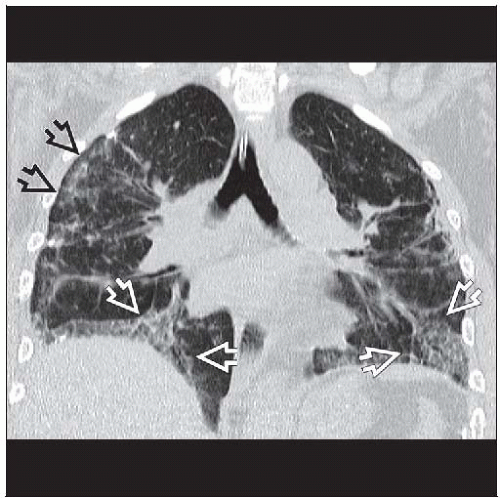
Ground-glass opacities > reticular opacities
Traction bronchiectasis out of proportion to reticular opacities
May have fine honeycombing: Microcystic honeycombing
Distribution: Lower (92%), diffuse (60%)
Peribronchovascular distribution, often fan-shaped
Subpleural sparing (20%)
Up to 30% over 3-year period evolve to more UIP pattern
Top Differential Diagnoses
Idiopathic Pulmonary Fibrosis (IPF)
Hypersensitivity Pneumonitis
Cryptogenic Organizing Pneumonia (COP)
Pathology
Etiology
Idiopathic
Scleroderma
Hypersensitivity pneumonia
Drug-induced lung disease: Amiodarone
Concept that NSIP represents early stage of UIP now discounted
Clinical Issues
Average duration of symptoms prior to diagnosis: 7 months
5 year survival > 80%
TERMINOLOGY
Abbreviations and Synonyms
Nonspecific interstitial pneumonia (NSIP)
Definitions
1 of the idiopathic interstitial pneumonias, less common than idiopathic pulmonary fibrosis (IPF), but with better prognosis than IPF
Distinguished by temporal and spatial uniformity of histologic findings
IMAGING FINDINGS
General Features
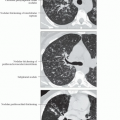
Best diagnostic clue: Traction bronchiectasis out of proportion to reticular opacities
Patient position/location: Peribronchovascular basilar distribution
Size: Generally involves 25-35% of lung volume
Morphology: Ground-glass opacities > reticular opacities
CT Findings
Morphology
Reticular opacities (85%)
Often admixed with ground-glass opacities
Ground-glass opacities, when present, usually exceed reticular opacities
Combined ground-glass opacities and reticular opacities may result in crazy-paving pattern
Traction bronchiectasis (80%)
Often out of proportion to degree of reticular opacities
Usually associated with considerable lobar volume loss
Mean 20% of total lung volume
Ground-glass opacities (75%)
Ground-glass opacities often exceed reticular opacities, especially early
Mean 25-35% of lung volume with ground-glass opacities
May have mosaic perfusion pattern (5%)
Peribronchial thickening (5%)
Honeycombing rare (5%) (so uncommon, should suggest usual interstitial pneumonitis [UIP])
May have fine honeycombing: Microcystic honeycombing
Isolated enlarged airspaces may be present (not clustered together like honeycombing)
Consolidation less common, mean 10% of lung volume
Distribution
Symmetry common
Craniocaudad
Lower (92%)
Diffuse (8%)
Axial
Diffuse (60%)
Peripheral (35%)
Subpleural sparing (20%)
Very thin rim
Bronchovascular distribution, often fan-shaped from hilum to lung periphery
Evolution
Up to 30% over 3-year period evolve to more UIP pattern
Reversible findings: Ground-glass opacities, reticular opacities, traction bronchiectasis
Adenopathy (80%)
Mediastinal lymph node enlargement < 2 cm short axis diameter
Most common location right paratracheal nodal station (American Thoracic Society [ATS] region 4) or subcarinal (ATS region 7)
Direct positive correlation between extent of parenchymal involvement and presence of lymphadenopathy
Accuracy
Should make confident diagnosis in 80% of cases
Positive predictive value of confident diagnosis = 70-80%
Radiographic Findings
Radiography
Radiography: Nonspecific, abnormal (90%)
Bibasilar interstitial thickening (“lace-like” honeycombing)
Imaging Recommendations
Best imaging tool: HRCT to detect and characterize interstitial lung disease
DIFFERENTIAL DIAGNOSIS
Idiopathic Pulmonary Fibrosis (IPF)
Honeycombing more common
Subpleural peripheral location, more common
No subpleural sparing
Ground-glass extent less common
Histopathology: Temporal inhomogeneity of lung injury
Poorer prognosis than NSIP
Hypersensitivity Pneumonitis
May have NSIP pattern
Occupational and home environmental history extremely important to discover known antigens
Mosaic perfusion pattern more common
Chronic disease usually more severe in mid and upper lung zones, honeycombing more common
Cryptogenic Organizing Pneumonia (COP)
Consolidation usually more prominent than in NSIP
May have multiple pulmonary nodules or single mass, uncommon with NSIP
Reverse halo sign not seen with NSIP
Reticular opacities uncommon but similar to NSIP
Desquamative Interstitial Pneumonia (DIP)
Smokers; smoking less common in NSIP
Diffuse ground-glass opacities with scattered cysts
Traction bronchiectasis uncommon
Pulmonary Alveolar Proteinosis (PAP)
“Crazy-paving” appearance, less common with NSIP
Central and geographic distribution, not bronchovascular or peripheral
No traction bronchiectasis
Sarcoidosis
Nodules: Most common pattern follows lymphatic pathways
Centrilobular, bronchovascular, and subpleural
Occasionally have predominant ground-glass opacities
Would not be associated with traction bronchiectasis if ground-glass opacities were predominant pattern
Adenopathy more common and nodes larger, especially in early disease
PATHOLOGY
General Features
General path comments: Spatial & temporal homogeneity, important to distinguish NSIP from UIP
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree