Use of imaging services in the emergency department (ED) continues to outpace the growth of actual ED patient visits. This imaging surge places the emergency radiologist at the forefront of acute care. Chest imaging constitutes a large percentage of examinations generated through the ED. In the time-sensitive acute care environment, efficiency is paramount; conventional radiographs are a common triage screening tool. It is not uncommon for the radiologist to “examine” the patient before the emergency physician does.
Navigating the multitude of acute and chronic diseases in the ED can be a daunting task for emergency radiologists. Applying a pattern-based approach and recognizing common disease states provides a road map for accurate and prompt diagnosis of chest emergencies. Understanding radiologic manifestations of these emergencies requires knowledge of the basic anatomy of the thoracic cavity.
Anatomic Considerations
Anatomic clues facilitate a pattern-based approach to image interpretation. Distinguishing whether opacity lies in the alveolar space, airway, or pleural space seems basic but is requisite for accurate diagnosis. Basic anatomic landmarks such as the bronchi, fissures, vascular and nonvascular mediastinal structures, pleura, and soft tissues are important when navigating conventional radiography, especially if only a single view is obtained.
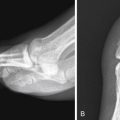
Not all opacities represent disease. Common anatomic variants such as pectus excavatum and benign masses can mimic acute disease. Recognition of these entities can help resolve diagnostic dilemmas. Displacement of fissures or mediastinal structures can be the first clue in recognizing lobar collapse or even pneumothorax. Left upper lobe collapse can be confounding and is often misinterpreted if secondary signs are not identified ( Fig. 9-1 ). Secondary signs include tenting of the diaphragm, hilar retraction toward the collapsed lung, and the Luftsichel sign (due to compensatory hyperinflation of the superior segment of the left lower lobe). When opacities abut and silhouette the pleura, the presence of obtuse or acute interfaces can help localize the lesion to the pleural or subpleural compartments, respectively. Accurate localization is vital in the setting of aspirated foreign bodies or penetrating trauma for surgical planning. Infiltrative diseases such as a necrotizing pneumonia or neoplasm can be multispacial. Thin-section computed tomography (CT), through the use of 1- to 2-mm collimation, provides exquisite anatomic detail as compared to conventional radiography but requires a greater understanding of segmental and subsegmental pulmonary anatomy to interpret the multitude of patterns that can be encountered.

Beyond lobar and segmental anatomy ( Fig. 9-2 ), the emergency radiologist should be familiar with the smallest unit visible on CT, which is the secondary pulmonary lobule. The walls of the secondary pulmonary lobule are composed of the interlobular septa. The interlobular septa are composed of connective tissue and house the pulmonary veins and lymphatic drainage to each lobule. Additional venolymphatic drainage is provided via the subpleural interstitium along the fissures and the periphery of the lung. At the center of each lobule is a bronchovascular bundle composed of a terminal bronchiole and pulmonary artery that supply oxygenated air and deoxygenated blood to the alveoli, where gas exchange occurs. They can be seen in disease states such as pulmonary interstitial edema when the septa become thickened due to vascular engorgement and increased hydrostatic pressure; radiographically these are manifested as Kerley B lines.

Pattern-Based Approach
Common patterns can be seen across varying disease states. For example, hematogenous spread of disease will manifest as randomly distributed nodules throughout the lungs regardless of cause. Therefore metastatic disease can mimic septic emboli. Cavitation can be seen in both metastatic disease and infection. Recognizing these blood-borne disease patterns helps to guide clinical treatment. Nodules in a perilymphatic distribution with septal involvement are more commonly associated with insidious disease processes such as sarcoidosis or lymphangitic carcinomatosis. Similarly, alveolar opacities, often characterized as ground-glass opacities, can represent a variety of disease states, including infection, hemorrhage, edema, neoplasm, or inflammation.
The commonly described tree-in-bud pattern seen on thin-section CT was first attributed to endobronchial spread of Mycobacterium tuberculosis (TB; Fig. 9-3 ). It described linear branching centrilobular opacities corresponding to impaction of distal airways. This finding is associated with other disease states where fluid, debris, pus, and mucus fill the distal bronchioles or if significant thickening of the distal bronchiolar walls is present. This pattern can indicate endobronchial spread of other bacterial, viral, or fungal infections such as cytomegalovirus and allergic bronchopulmonary aspergillosis (ABPA). The tree-in-bud pattern can also be seen in the setting of chronic airway disease states such as cystic fibrosis, after toxic inhalation or aspiration. Correlation with clinical and laboratory data is necessary when there is severe underlying chronic airways disease to exclude superimposed acute infection.

Several other common patterns of pulmonary disease can be seen in the acute setting. The “crazy-paving” pattern ( Fig. 9-4 ) seen on thin-section CT is characterized by scattered or diffuse ground-glass attenuation with superimposed interlobular septal thickening. This was initially described in acute pulmonary alveolar proteinosis but subsequently has been reported in a wide range of infectious and inflammatory diseases such as Pneumocystis jiroveci pneumonia ( Fig. 9-5 ), acute respiratory distress syndrome (ARDS), organizing pneumonia, and even pulmonary edema. The presence of this pattern should prompt the radiologist to alert the clinician about possible underlying immunodeficiency disorders, most commonly acquired immunodeficiency syndrome (AIDS). Another commonly encountered pattern is “mosaic” perfusion of the lungs, which can be seen in chronic thromboembolic disease, small airways disease with air trapping, and hypersensitivity pneumonitis (HP).


Cavitary masses are commonly associated with fungal and tuberculous infections. Community-acquired pneumonias can also be complicated by necrosis and cavitation. In adults cavitary lesions typically contain mixed flora, including anaerobes such as Klebsiella . In children staphylococcal pneumonia is the most common cause of cavitary pneumonia. Primary lung neoplasm and metastatic disease may cavitate and are usually included as differential considerations particularly in smokers or patients with known primary malignancy. Computed tomography can be important for additional characterization to exclude bronchopulmonary abscess and empyema.
Clinical Considerations
For the emergency radiologist, acquiring accurate history can be a challenge because patients are often triaged quickly before relevant clinical information is obtained or available. Vague clinical indications such as “cough,” “chest pain,” or “shortness of breath” are commonplace. Direct communication with clinical providers can yield important data that can augment radiologic interpretation. The added history of chronic disease states such as chronic renal failure, sickle cell disease, or malignancy sheds light on imaging findings. Clinical data regarding acute exposure to inhalational toxins, thermal injury, or patient’s immune status allow the radiologist to refine his or her interpretation. Reviewing basic laboratory data and patient medical records is frequently a worthwhile endeavor that can yield information unbeknownst even to the clinician.
Recognizing parenchymal lung disease patterns of susceptible populations can alert the radiologist to assess for associated potential complications. For example, sickle cell anemia is a frequently encountered disease in the acute care setting. Patients are predisposed to infection, as well as bouts of acute vasoocclusive crises known as acute chest syndrome . Even without history, the recognizable radiographic stigmata of sickle cell disease such as osteonecrosis and cardiomegaly can alert the radiologist to assess for changes suggestive of acute chest syndrome—which is defined as the presence of a new consolidation on chest radiography ( Fig. 9-6 ). Sickle cell patients are 100 times more susceptible to pneumonia than the general population, with a 30% recurrence rate. Pulmonary edema related to left ventricular failure can also be seen. Chronic findings of pulmonary hypertension related to microvascular occlusion and chronic hypoxia are manifested as scarring, architectural distortion, and enlargement of the pulmonary arteries.

Patients with obstructive lung physiological findings, most commonly long-time smokers, appear to have hyperinflated lungs because of pulmonary emphysema and are predisposed to infection and neoplasm. Parenchymal damage due to cystic or bullous changes can predispose this population to secondary spontaneous pneumothorax. Another commonly encountered entity is cystic fibrosis. Patients present with frequent respiratory tract complaints, including cough, dyspnea, and fever. The radiographic findings of cystic fibrosis include bronchiectasis and parenchymal scarring. Computed tomography findings include bronchial wall thickening, progressive bronchiectasis, and mucoid plugging within the bronchi with upper lobe predominance. Hyperinflation and secondary spontaneous pneumothorax can also be seen. Consideration should also be given to the wide range of disease states that result in immunosuppression. In addition to human immunodeficiency virus (HIV)/AIDS, patients undergoing chemotherapy, long-term corticosteroid use, or recent organ transplantation are susceptible to opportunistic infections.
Infection
Infectious disease processes of the thoracic cavity are diverse in clinical and radiologic presentation depending on the pathophysiological characteristics of the offending organism. Lobar and interstitial pneumonias, cavitary pneumonias, endobronchial infection, pleural-based infections, and hematogenous spread of infection are all encountered in the acute care setting, and the emergency radiologist should be alert to the radiographic findings and common associations of these disease states.
Community-Acquired Pneumonia
Community-acquired pneumonia is a common cause of significant morbidity and mortality, especially in older adults. Mycoplasma , Streptococcus , and viral respiratory pathogens account for the majority of community-acquired pneumonias encountered. Radiographic findings vary from normal to patchy, or confluent air-space opacities with air bronchograms to occasional progression to cavitation. Opacities within the lingula and right middle lobe may silhouette the cardiac margins. Lower lobe opacities may obscure the diaphragm or appear as a retrocardiac density. Differentiating viral from bacterial pneumonias on radiography is rarely possible; however, interstitial patterns with lymphadenopathy tend to typify viral pneumonias, whereas bacterial pneumonias are more likely to be lobar in distribution with occasional effusions and cavitation. Radiographic follow-up is beneficial to exclude underlying lung pathologic conditions after treatment.
Although CT is more sensitive than radiography in detecting community-acquired pneumonia, practical issues of radiation, time, and cost argue against routine use unless complication such as abscess formation is suspected. Computed tomography imaging findings vary greatly depending on route of spread. The most common finding on CT is air-space consolidation with or without bronchograms. Other common findings include centrilobular nodules in viral and Mycoplasma pneumonias. Lymphadenopathy and effusions can also be seen. Airway-predominant disease may show bronchial wall thickening, bronchocentric ground-glass opacities, and tree-in-bud type opacities.
Atypical Infections
Atypical pneumonia is a nonspecific term that includes rare and opportunistic infections caused by fungal, viral, and bacterial pathogens typically encountered in patients with comorbidities such as AIDS or immunosuppression related to transplant or chemotherapy and in longtime smokers. Chronic debilitating diseases such as renal failure, diabetes, sickle cell disease, and cystic fibrosis may also render patients prone to atypical infections. The emergency radiologist should be familiar with common radiographic manifestations of these infections because prompt and accurate diagnosis can greatly affect the clinical course.
Pulmonary Tuberculosis
Risk factors for pulmonary TB include acquired, innate, or iatrogenic immunodeficiency and exposure to infected individuals. Prisoners and recent immigrants from countries where TB is endemic have higher risks for infection. Primary TB may manifest as lymphadenopathy, parenchymal disease, miliary disease, or pleural effusion. Multiple patterns can be seen concomitantly. Classic findings of reactivation TB include a predilection for the upper lobes, cavitation, and relative absence of lymphadenopathy; however; recent studies report overlapping radiographic findings in primary and postprimary TB.
Miliary TB is found mainly in immunocompromised patients with disseminated disease affecting multiple organ systems (see Fig. 9-3 ). Distinct radiographic findings consist of innumerable, 1- to 3-mm diameter nodules uniformly distributed throughout both lungs with slight lower lobe predominance. High-resolution CT depicts nodules in a random distribution. These nodules resolve with treatment, but they may coalesce to form focal areas of consolidation. Thickening of interlobular septal networks is frequently present. Diffuse or localized ground-glass opacity may indicate superimposed ARDS.
Cavitary TB is a hallmark of reactivation TB (see Fig. 9-3 ). Typical cavities are irregular and thick walled and can occur within surrounding areas of consolidation. Cavitary neoplasms or fungal infections should also be considered in the differential diagnosis depending on the clinical scenario. Complications of cavitary lesions include rupture into pleural space and bronchopleural fistula. A small percentage of cavities showing air-fluid levels may indicate the presence of superinfection.
Computed tomography findings of bronchocentric TB are circumferential mural thickening and luminal narrowing, which may cause lobar collapse or hyperinflation, obstructive pneumonia, and mucoid impaction. In active disease the airway lumens are irregularly narrowed and have thick walls, whereas in fibrotic disease the airways are smoothly narrowed and have thin walls. Tree-in-bud opacities and traction bronchiectasis of the upper lobes may be evident (see Fig. 9-3 ). Radiographic evidence of lymphadenopathy is seen in up to 90% of children and 50% of adults. Adenopathy is more often unilateral and tends to be right-sided, involving the paratracheal and hilar regions, but may be bilateral in 30% of cases. Nodes larger than 2 cm tend to have low-attenuation centers because of necrosis and are highly suggestive of active disease. Nontuberculous mycobacterial species such as Mycobacterium avium-intracellulare complex can manifest as chronic right middle lobe and lingular bronchiectasis and tree-in-bud opacities.
Fungal Infections
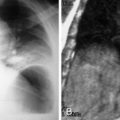
Fungi are uncommon pathogens in otherwise healthy populations. Fungal infections occur more frequently in susceptible populations. Fungal infections can manifest with unique radiologic findings. Pulmonary aspergillosis is an opportunistic infection that has four distinct forms: ABPA, aspergilloma, and invasive and semi-invasive aspergillosis. ABPA can be seen in long-standing asthmatic patients typically under the age of 40 years. Eosinophilia and elevated serum immunoglobulin E levels are features of ABPA. During the initial stages of ABPA, chest radiographs may appear normal or depict asthmatic changes. If mucoid impaction within the dilated central bronchi is present, the classic “finger-in-glove” appearance may be seen ( Fig. 9-7 ). Computed tomography findings include fleeting alveolar opacities, central upper lobe bronchiectasis, bronchial wall thickening, and mucoid impaction. Lung distal to the impacted region may become hyperinflated or atelectatic.