potential radiation risks are lower than in the axial skeleton, which is located near more radiosensitive mediastinal, abdominal, and pelvic organs.12 More recently, selective use of fetal CT with 3D reformations has been described in cases in which US and genetic data are inconclusive to either diagnose or exclude a suspected skeletal dysplasia with impact on accurate counseling of families.5,12,13,14,15
 FIGURE 21.1 Normal bone marrow conversion in long bones. Sequential age-related changes in hematopoietic marrow to fatty marrow distribution are illustrated in this figure representing a femur. |
development of a secondary ossification center and progressive conversion into bone.
of long bones, also guides the reparative response to stimuli (e.g., trauma, infection, neoplasm, metabolic disorder, and nutritional status). In children, the periosteum is physiologically more active and less adherent to the cortex than in adults, allowing for earlier and more aggressive appearance of the periosteal reaction.26 The differential diagnosis for benign periosteal reaction in the pediatric population is summarized in Table 21.1.
 |
TABLE 21.1 Differential Diagnosis of Physiologic Periosteal Reaction (1-6 Months of Age) | |||||
|---|---|---|---|---|---|
|
bony abnormalities in the axial and appendicular skeleton of affected pediatric patients. This information should be integrated with the rest of the information provided by a multidisciplinary team approach in order to reach a correct diagnosis. A comprehensive analysis of imaging findings in skeletal dysplasias is beyond the scope of this chapter. However, the most common skeletal dysplasias identifiable through imaging at birth or later in life are summarized in this chapter. In addition, the most common disorders of limb reduction, congenital bowing of the legs, and congenital foot deformities are reviewed. Finally, syndromic skeletal abnormalities, developmental hip dysplasia, and skeletal abnormalities associated with neuromuscular disorders are discussed (Table 21.2).
TABLE 21.2 Selected Spectrum of Skeletal Dysplasias with Identifiable Imaging Findings at Birth and Later in Life | ||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||
posterior vertebral scalloping, narrow chest with short ribs, rhizomelia with short thick tubular bones and metaphyseal flaring, notched physis (V shaped), short metacarpals and phalanges in “trident” configuration (separation between third and fourth fingers), horizontal acetabular roof, and squared iliac wings (“tombstone configuration”) (Fig. 21.8). Prenatal US demonstrates short long bones, particularly femurs and humeri, flat vertebral bodies, and large skull. In addition, small chest, short fingers, and polyhydramnios have been described.46
the onset of symptoms ranges from infancy to early childhood rather than at birth. The most common types of metaphyseal chondrodysplasia are Schmid, McKusick, and Jansen variants.
with apex extending away from the joint (Fig. 21.10). The osteochondromas can also be seen in flat bones, hands, ribs, and spine. There is the potential risk for malignant transformation in ˜5% of affected patients with multiple cartilaginous exostoses, most commonly chondrosarcoma.62
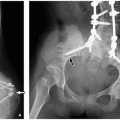
of an extremity with limping, and osseous deformities with possible pathologic fractures.64
and malignant). Musculoskeletal manifestations of NF1 include skull abnormalities (sphenoid wing dysplasia, erosions, and enlargement of foramina), scoliosis and kyphosis, pseudarthrosis of long bones (tibia most common), osteoporosis, and short stature (Fig. 21.15).72
 FIGURE 21.14 Neurofibromatosis type 1. Plexiform neurofibroma, diffusely expanding a peripheral nerve and its branches (upper panel), is seen. The cut surface (lower left) is tan white and firm, resembling fibrous tissue. In this example, the majority of the mass has the histologic appearance of neurofibroma (lower center), whereas areas of increased cell density and round “epithelioid” cells characterize a component of malignant peripheral nerve sheath tumor (lower right). (Hematoxylin and eosin, original magnification, 400×.)
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|