Case presentation
A 17-year-old male presents with right-sided facial numbness and extremity weakness that has been intermittent for the past 1 month. Several hours ago, the patient noted he had difficulty ambulating when rising out of bed due to the weakness, causing him to fall. He was able to prevent injury by steadying himself on a chair. He went back to lie down and then called his mother, who noted that he had slurred speech. He was taken to an outside facility after being transported by emergency medical services. There, he was told he was “fine” and was discharged home. His mother then brought him for evaluation to your department after he felt weak and was having slurred speech again; he also was noted to have a right facial droop. He denies visual changes, ataxic episodes, altered consciousness, recent illness, and recent trauma. He has no significant medical or familial history.
Physical examination reveals a well-appearing patient in no distress. He has age-appropriate vital signs including his blood pressure. His physical examination is remarkable for right facial droop, tongue deviation to the right, and right upper extremity weakness. He has no other neurologic deficits.
Imaging considerations
Pediatric patients presenting with symptoms concerning for a stroke or who have previously had a stroke and there is concern for recurrence should undergo imaging as soon as possible. There are several options available to the clinician.
Computed tomography (CT)/CT angiography (CTA)/CT venography (CTV)
This imaging modality is rapid and available but does involve exposure to ionizing radiation. Unenhanced CT is useful in detecting hemorrhage and changes of ischemic stroke within 6 hours after the onset of the ischemic process, although prior to this, the study may be unremarkable. , The sensitivity and specificity of CT for pediatric stroke have not been validated in studies, but expert opinion for its use has been based on extrapolation and application from existing adult data. Non—contrast CT is the initial imaging modality in patients with a history and examination concerning for hemorrhagic and, in some cases, ischemic stroke.
CTA and perfusion studies may also be performed in children with suspected ischemic events and are often utilized in patients in whom magnetic resonance imaging (MRI) might be contraindicated, such as those with cochlear implants or other medical devices that are not MRI compatible. CTV is very useful in detecting cavernous sinus thrombosus. ,
MRI/MRI-angiogram (MRA)–venogram (MRV)
MRI is an excellent modality for imaging patients with concerns for an ischemic event. A typical standard stroke protocol includes fluid attenuated inversion recovery (FLAIR), diffusion-weighted imaging, and susceptibility-weighted imaging (SWI) and T1 sequences. Certain sequences of MRI are highly sensitive in the detection of parenchymal hemorrhage, subarachnoid hemorrhage (FLAIR and SWI sequences particularly), and, using perfusion techniques, cerebral blood flow, cerebral blood volume, and transit times. , , MRA and MRV can be used to evaluate cerebral blood vessel patency and evaluate vascular anatomy, and vascular imaging should be obtained when the cause of stroke is suspected to be ischemic or hemorrhagic. MRI is more sensitive than CT for acute ischemia and is better at evaluating the posterior fossa and can be useful in diagnosing stroke mimics.
The International Pediatric Stroke Study Neuroimaging Consortium recommends MRI as a first-line imaging study. , Due to the time required to perform the study, sedation, especially in younger patients, may be required. There are fast-acquisition protocols that some institutions have implemented, but this is still a 20-minute study, and sedation, even when these protocols are utilized, may still be required.
Ultrasound (US)
This modality is useful in neonates or infants with open fontanelles, allowing for imaging of the cerebral parenchyma, although visualization of the posterior fossa may be limited. Ischemic injury of the parenchyma may be seen as areas of increased echogenicity with mass effect. However, US has been shown to be less sensitive compared to CT and MRI in detecting ischemic lesions. , While this limits the usefulness of cranial US in detecting ischemia, the availability, lack of ionizing radiation, and portability of this modality make it useful in neonates to evaluate parenchymal hemorrhage, intraventricular hemorrhage, and gross anatomic evaluation of the cerebrum in neonates.
Catheter angiography
Cerebral angiography is useful in evaluating vascular malformations and is an accurate modality to evaluate the cerebral vasculature, providing great detail about blood vessels, including smaller vessels and branches, although the likelihood of identifying a vascular abnormality in patients with a normal MRI scan is low. , Angiography can also be therapeutic if neurointerventional radiology specialists are available. However, there are several limitations to the utilization of this test. Catheter angiography is often available only at specialty pediatric centers and sedation may be required in young children since the risk of arterial injury during the procedure is higher in uncooperative children.
Imaging findings
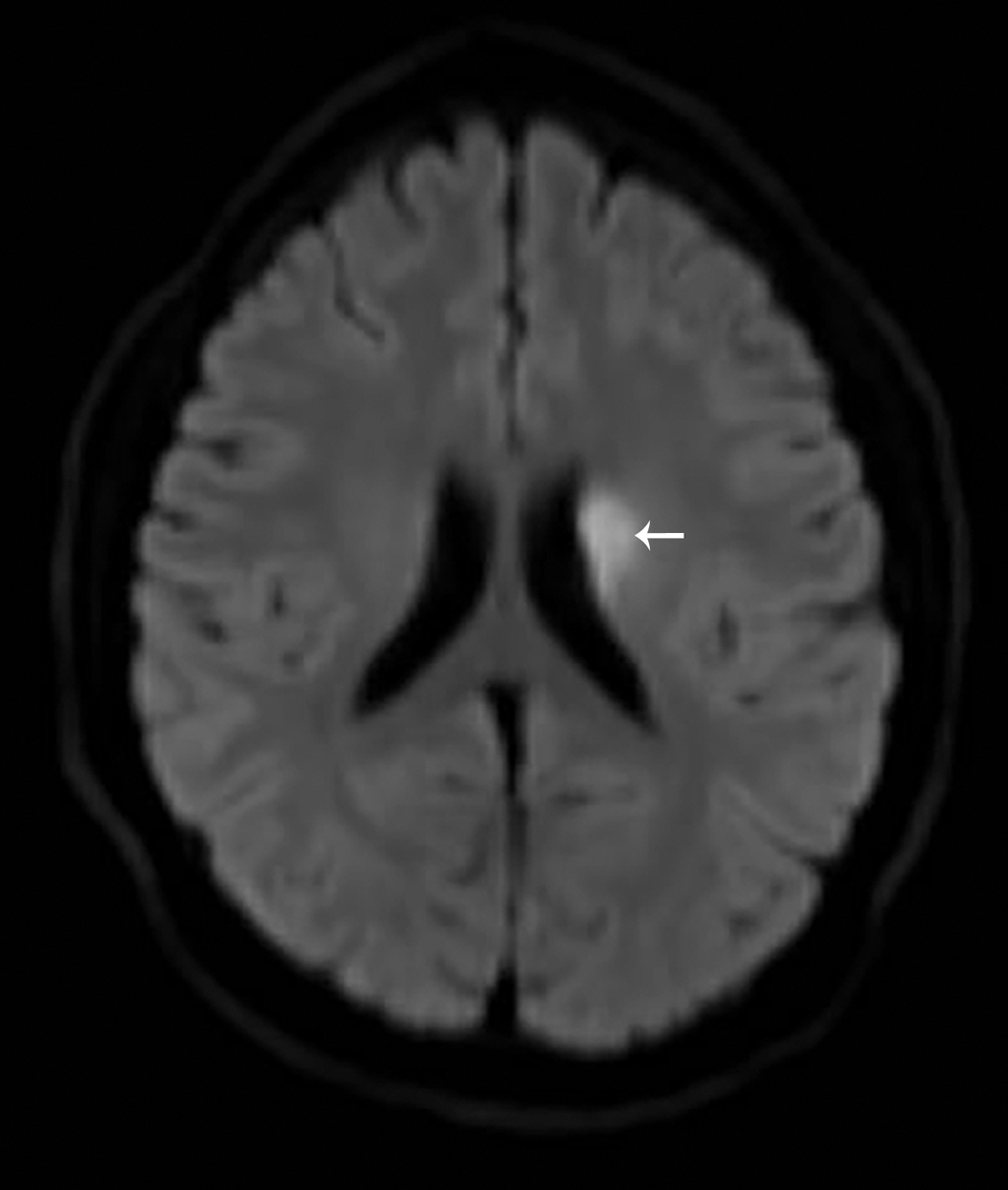
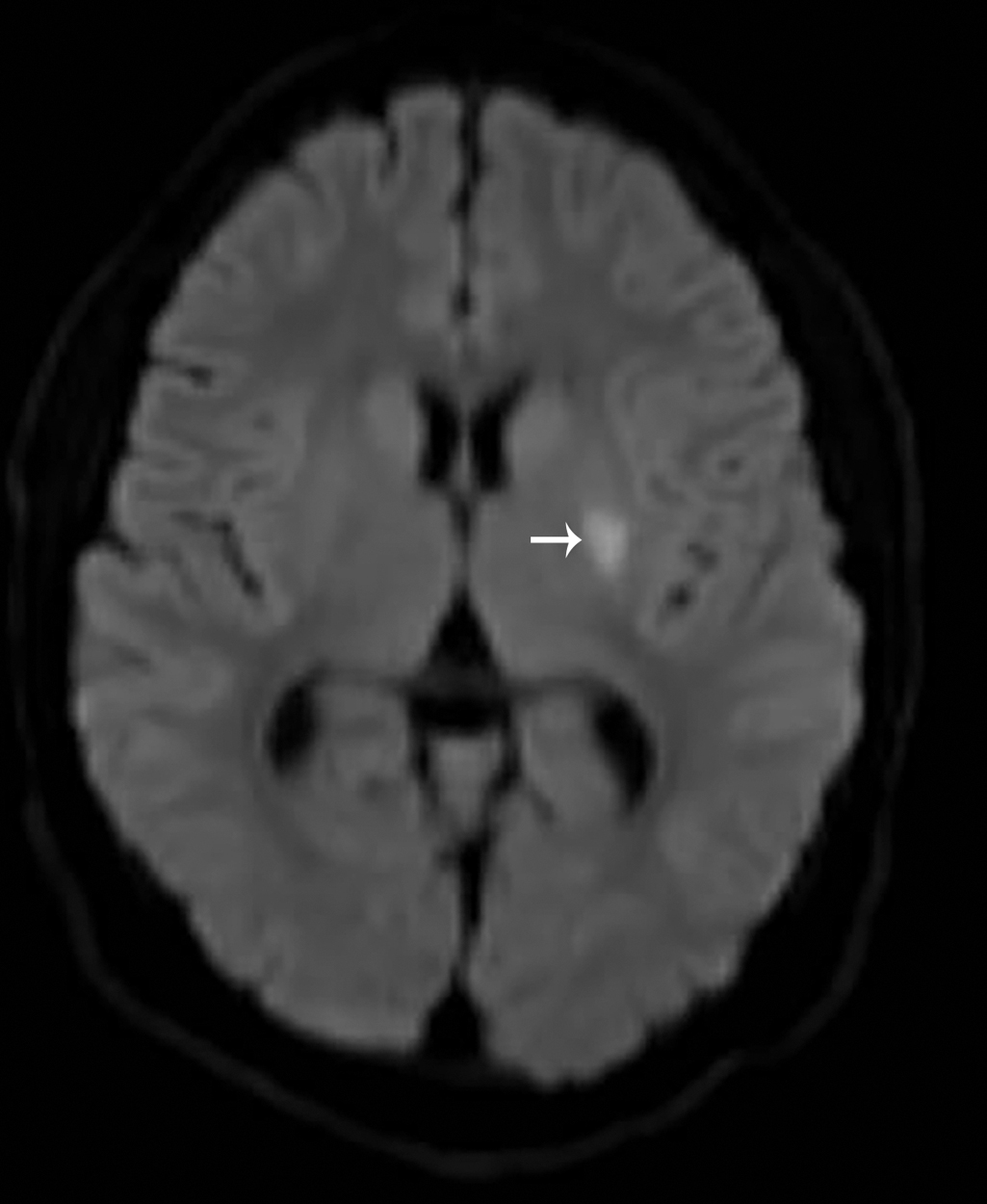
With the history and physical examination, the decision was made to obtain imaging. An MRI study of the brain, with and without intravenous contrast, was obtained. Selected images from that study are provided here. These images demonstrate a focal area of restricted diffusion involving the left corpus striatum and left corona radiata without evidence of acute hemorrhage and a suspected chronic lacunar infarct within the left thalamocapsular region ( Figs. 30.1 and 30.2 ).
Case conclusion
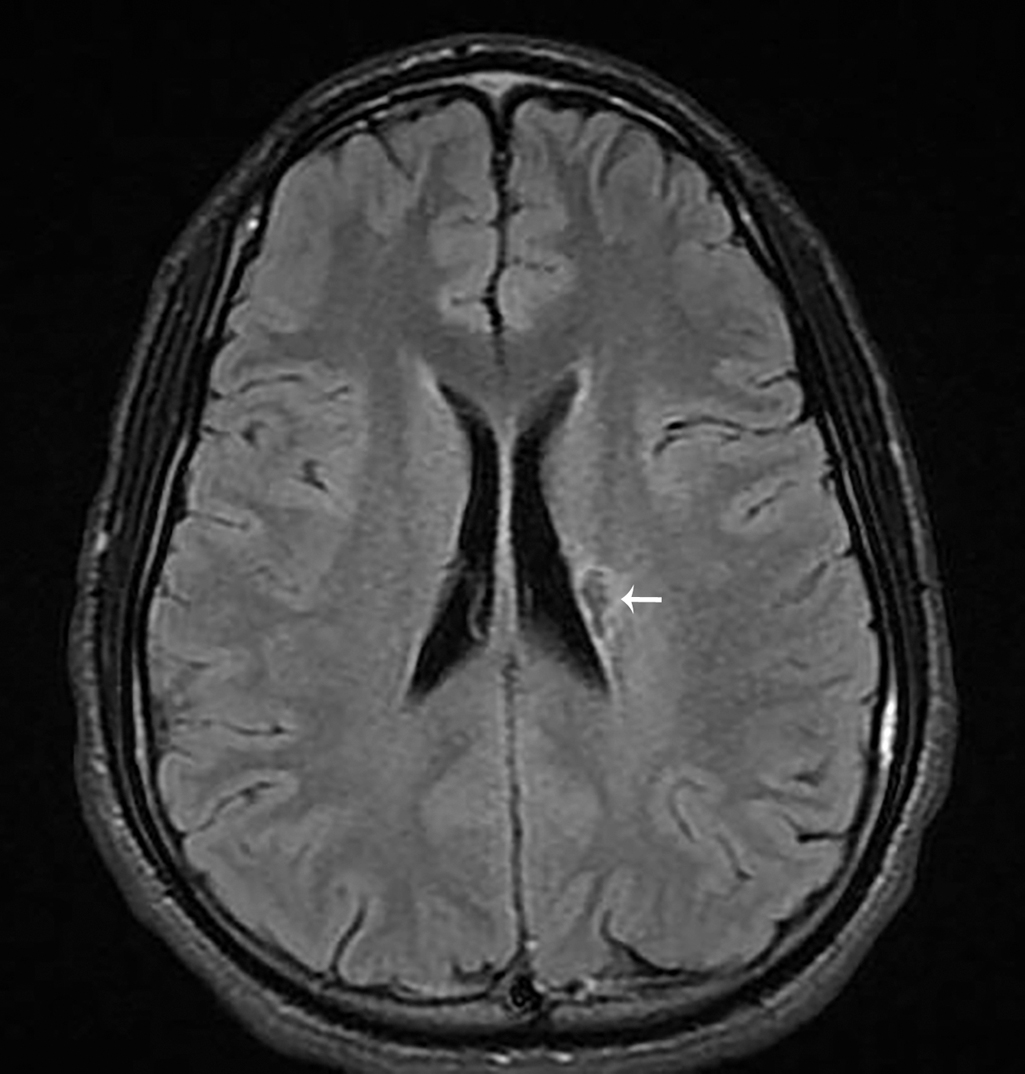
Pediatric Neurology was consulted. The patient also underwent CTA of the head and neck, which were normal. The patient was initially started on unfractionated heparin and Pediatric Hematology was also consulted. After extensive discussion between the family and the consultants, the patient was transitioned to aspirin. His symptoms resolved within 48 hours. He was carefully monitored by Pediatric Neurology and Pediatric Hematology and had no further symptoms. Follow-up imaging (selected images provided here) demonstrates encephalomacia and gliosis in the location of previous acute infarction ( Figs. 30.3 and 30.4 ).
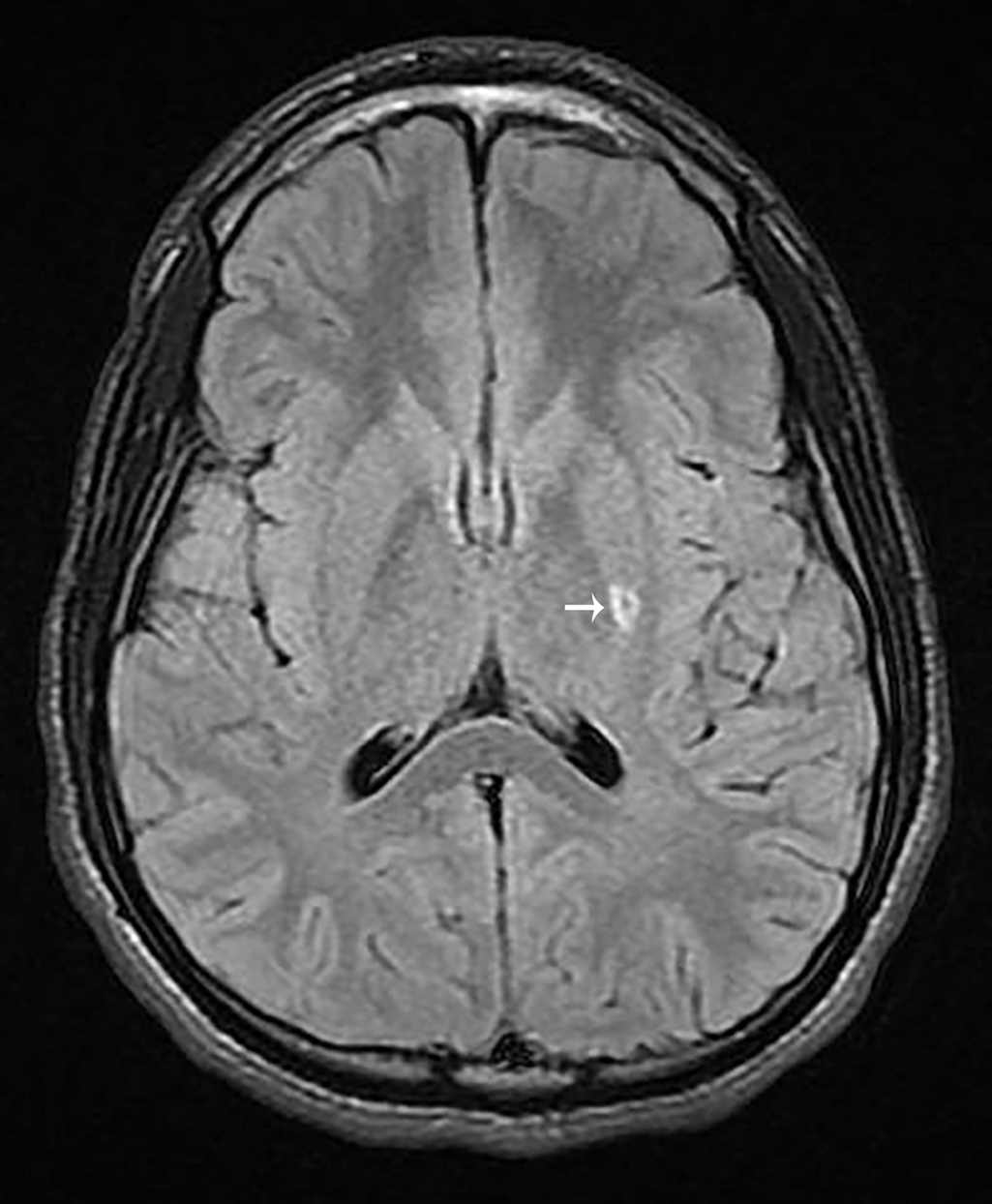
Stroke is a cerebrovascular event that may be ischemic or hemorrhagic. While approximately 80% of adult strokes are ischemic, only approximately 55% of pediatric strokes are ischemic. , The incidence of pediatric stroke has been reported to be 2–3/100,000 children per year in the United States. , Over one-half of pediatric patients who have suffered a stroke will have cognitive and motor disabilities and up to one-third will have recurrent stroke. Lacunar infarctions are not commonly seen in the pediatric population. Lacunar strokes involve a branch of a cerebral artery that, when occluded, produces a small noncortical infarction. These branches originate from the Circle of Willis, middle cerebral artery, or the basilar artery.
There are comorbidities that are associated with ischemic and hemorrhagic stroke in pediatric patients. These include sickle cell disease, vascular anomalies, hypertension, congenital heart disease, head and neck trauma, coagulation disorders, and infection. , , , Vascular wall injuries are also a risk factor for ischemic stroke in this population. About one-half of pediatric patients have a previously identified risk factor for stroke at the time of infarction, and after a complete evaluation, up to two-thirds have more than one identifiable risk factor; however, up to one-third of pediatric patients with stroke do not have an apparent underling etiology. , ,
Sickle cell disease
Patients with sickle cell disease carry a higher risk of stroke compared to the general population. In sickle cell patients with stroke, 60% to 90% will have abnormalities of the cerebral vasculature on angiography or MRA, including stenosis and occlusion. , ,
Vascular anomalies/aneurysms
Symptomatic aneurysms are not common in pediatric patients. Cerebral aneurysms can be associated with genetic conditions, such as Ehlers-Danlos syndrome, coarctation of the aorta, and autosomal dominant polycystic kidney disease. Pediatric patients may present with subarachnoid, intraventricular, or intracerebral hemorrhage, and vascular anomalies are present in a wide age range, including newborns. One study demonstrated that 70% of children with vascular anomalies had recurrent stroke, whereas children without vascular anomalies had none.
Focal cerebral arteriopathy
One of the most common causes of stroke in a previously healthy child is focal cerebral arteriopathy of childhood, or FCA, which is also known as transient cerebral arteriopathy. The exact pathophysiology is not well understood. This is an acute, monophasic disease causing unilateral stenosis of the intracranial cerebral arteries, mainly involving the anterior circulation and places the child at high risk for recurrent stroke (up to 25% within 1 year). Herpes viruses, especially varicella and herpes virus simplex 1, have been identified as a possible trigger of FCA, secondary to inflammation of the walls of affected arterial segments. , Pediatric patients with FCA can have rapid progression over days to weeks, and rapid progression is associated with a risk of recurrent ischemia. , , Patients with enhancing arteriopathies on vessel wall imaging have been shown to have a higher risk of arteriopathy progression than those without enhancement. , ,
Hypertension
Hypertension is a known risk factor for stroke in the adult population and may be a risk factor for stroke in pediatric patients. , , , One case series of pediatric patients demonstrated a significant association between blood pressure greater than the 90th percentile and cerebral arterial abnormalities.
Cardiac disease
Pediatric patients with complex congenital heart lesions with right-to-left shunting and cyanosis are prone to stroke, but stroke can be associated with any heart lesion. Stroke is more common among patients with uncorrected heart disease. , Stroke may also be seen in patients with valvular heart disease as a result of infection (e.g., rheumatic fever), those with prosthetic heart valves, and as a complication of cardiac catheterization procedures.
Trauma
Carotid artery or vertebral artery dissection as a result of a traumatic injury is a recognized risk factor for stroke.
Hematocrit abnormalities/coagulopathies
Abnormal hematocrit is a risk factor for the development of stroke. Patients with low hematocrit (commonly seen in pediatric patients with iron deficiency) are at high risk for arterial stroke, while those with an elevated hematocrit are at risk for central venous thrombosis. Coagulation disorders and thrombocytopenia are risk factors for pediatric hemorrhagic stroke, and the degree of severity of the underlying disorder correlates with risk of hemorrhage. , In a review of the association between stroke and iron deficiency anemia, previously healthy children with stroke were significantly more likely to have iron-deficiency anemia and children with iron deficiency anemia accounted for more than half of stroke cases in the study population (children 12 to 389 months of age). A case control study found previously healthy children with stroke were 10 times more likely to have iron-deficiency anemia than healthy children without stroke and children with iron-deficiency anemia accounted for more than half of all stroke cases in children without an underlying medical illness.
Infection
In the pediatric population, stroke has been associated with infection. Recent varicella infection has been shown to be associated with stroke involving the middle cerebral artery. , Patients with prior immunodeficiency or leukocytosis are at risk for stroke as well. , In patients with otitis media and meningitis, dural sinus venous thrombosis may be a complication, although this entity is not exclusive to infectious processes and may be a result of other underlying conditions, including autoimmune diseases, anemias, prothrombotic disorders, and metabolic conditions. Intracranial complications from infection may present with subdural effusion or hematoma, subarachnoid hemorrhage, or intracerebral hemorrhage; often, clinical signs are nonspecific, such as seizure and headache. ,
The NIH Pediatric Stroke Scale (PedNIHSS) is an adaptation of the adult NIH Stroke Scale (NIHSS) and designed for use in children 2 years and older with concerns for ischemic stroke (hemorrhagic stroke has not been validated). The NIHSS has been used to quantitate stroke severity and outcomes at 7 and 90 days. , The PedNIHSS closely resembles the NIHSS. A multicenter cohort study found that the PedNIHSS demonstrated excellent interrater reliability and may be predictive of future disability. , An online tool is available to calculate this score at https://www.mdcalc.com/pediatric-nih-stroke-scale-nihss .
The management of pediatric stroke depends on the underlying etiology. It is important to note that there are no standardized treatment protocols for the treatment of acute pediatric stroke. A notable exception to this is the management of sickle cell disease patients. Stroke in these patients is managed with hydration, correction of underlying metabolic derangements (such as hypoxemia), and exchange transfusion. , , In general, acute management of pediatric stroke may include the use of antiinflammatory medications (such as aspirin or steroids) and antithrombotic therapies (such as low-molecular-weight heparin or warfarin). , Treatment strategies are based on limited pediatric studies, expert opinion, anecdotal evidence, and adult studies; they are designed to treat the immediate stroke but also to prevent recurrence, which occurs in up to 25% of patients. Regardless of the etiology of the ischemic event, management includes providing oxygen for hypoxemic patients, maintaining normothermia, addressing electrolyte abnormalities, providing rehydration, treating anemia, and treating seizures, although there is no strong evidence that routine administration of antiepileptic medications for seizure prophylaxis in the absence of seizures is beneficial. Hypothermic measures are not recommended. Studies have indicated that having an established pediatric stroke protocol benefits patients with rapid diagnosis, including decreased time to imaging and decreased time to treatment. ,
Thrombolytic therapy is a particularly interesting area of pediatric ischemic stroke treatment. Low-molecular-weight heparin is preferred over unfractionated heparin because it is easier to administer and monitor but has the disadvantage of being more difficult to reverse rapidly. , Both of these medications are used for short-term anticoagulation; long-term anticoagulation can be achieved with low-molecular-weight heparin or warfarin. , Aspirin may be another option in patients without sickle cell disease who do not have a hypercoagulable disorder. Tissue plasminogen activator (tPA) has not been well studied in pediatric patients. There have been case reports of its use in pediatric patients. It may be reasonable to consider use of tPA if certain criteria are met, such as persistent disabling neurologic deficits or radiographically confirmed cerebral large artery occlusion. Until more definitive data are available, routine use of tPA is not recommended and consultation with a pediatric neurologist is reasonable. Endovascular thrombectomy has also been explored as an acute thrombolytic therapy and may be useful in the pediatric population, given concerns about introducing catheters into small groin and cerebral arteries, size-based limitations on contrast dye, and radiation exposure. Since safety and efficacy data are lacking and there are no definitive guidelines regarding thrombectomy in pediatric patients, consultation with an expert in the care of pediatric stroke is reasonable if this therapy is being considered. Aneurysms in pediatric patients are treated much in the same manner as in adults. Microsurgical and endovascular techniques are utilized. , , Consultation with Pediatric Neurosurgery can be helpful in the management of these patients.
Corticosteroids are used with increasing frequency in children with FCA, although the evidence for this is not abundant. , One study in particular demonstrated improvement in children with FCA treated with a combination of steroid and antithrombotic therapy versus antithrombotic therapy alone, finding lower levels of neurologic impairment in the combination therapy group. However, one must balance the possible benefits of the use of corticosteroid therapy with the potential worsening of an existing infectious process.
Management of hemorrhagic stroke is extrapolated from adult studies. Generally, management or blood pressure to prevent hypotension, strategies to prevent increased intracranial pressure, and hyperosmotic agents (hypertonic saline) are utilized. Prevention of further bleeding can be done with transfusion of platelets, clotting factors, fresh frozen plasma, or vitamin K, depending on the etiology of the bleeding. Reversal of antithrombotic therapy may be indicated and there are a variety of agents available for this purpose. If clinically indicated, surgical craniectomy can be performed when there is a need for decompression (brainstem compression, bleeds >3 cm, hydrocephalus). ,