(1)
Department of Nuclear Medicine, Kuwait University, Safat, Kuwait
10.1 Tumor Pathology
10.1.1 Biological Behavior
10.1.2 Tumor Grading
10.1.3 Tumor Staging
10.1.4 Tumor Growth Rate
10.2 Tumor Biology
10.2.1 Cell Growth and Cell Cycle
10.2.4 Invasion and Metastasis
10.2.5 Carcinogenesis
10.2.6 Apoptosis
10.2.7 Hereditary Cancer
10.3.2 Scintigraphic Imaging
Abstract
The classification and typing of tumors depend mainly on the histopathological diagnosis, which is made on the basis of gross and microscopic examination of tissues. Tumor classification is based on histogenesis, degree of cellular differentiation (i.e., well or poorly differentiated), and biological behavior (benign vs. malignant).
10.1 Tumor Pathology
The classification and typing of tumors depend mainly on the histopathological diagnosis, which is made on the basis of gross and microscopic examination of tissues. Tumor classification is based on histogenesis, degree of cellular differentiation (i.e., well or poorly differentiated), and biological behavior (benign vs. malignant).
All tumors, whether benign or malignant, have two components: (1) proliferating neoplastic cells and (2) supportive stroma, which is host derived and made up of connective tissue and blood vessels. While the neoplastic cells determine the nature of the tumors, tumor growth and evolution depend on the stroma [1].
In the past, the general concept was that neoplasms of certain phenotypes arise from their normal cell counterpart. However, evidence that accumulated over the years has proven the inaccuracy of this histogenetic assumption. It is now believed that most tumors arise from immature cells that can transform and acquire phenotypic features similar to those of one or more normal cell types. For example, rhabdomyosarcomas are tumors that show rhabdomyoblastic differentiation rather than tumors that arise from striated muscle cells [2]. In some instances, the immature cells can undergo divergent differentiation into two cell types, as in the case of mixed tumors of the salivary gland (Fig. 10.1), or they have the capacity to differentiate into any adult cell type, as in teratoma.
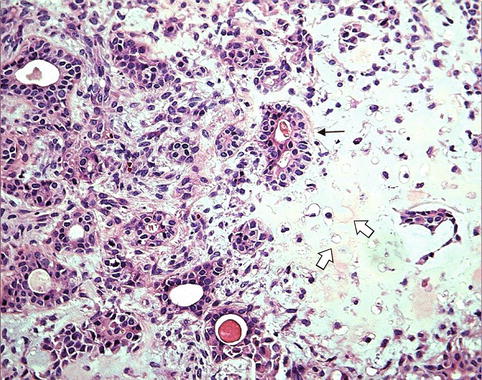

Fig. 10.1
Benign mixed tumor of salivary gland (pleomorphic adenoma). The tumor consists of an epithelial component “glands” (arrow) and a mesenchymal component “cartilage” (open arrows)
10.1.1 Biological Behavior
The categorization of tumors into benign and malignant is an oversimplification of the wide behavioral range of neoplasms. There are tumors that exhibit intermediate behavior. This has led to the introduction of a third category designated as “borderline or undetermined,” which represents low-grade malignant tumors that can mostly be managed by conservative therapeutic approach. The best examples are borderline tumors of the ovary and uterine smooth muscle of low malignant potential. Currently, the malignant category is restricted to tumors that have metastatic properties.
10.1.1.1 Benign Tumors
A tumor is considered benign when its gross and microscopic characteristics are relatively innocent, implying that it will remain localized and cannot spread to other sites. A benign tumor is generally composed of well-differentiated cells that resemble their normal counterpart. In general, the addition of the suffix -oma to the cell of origin describes benign tumors; for example, adenoma indicates a benign tumor of epithelial cell origin. Tumors that arise from mesenchymal tissues are designated according to their putative cell of origin (e.g., fibroma, chondroma, lipoma, and leiomyoma). Benign tumors can also be classified on the basis of their macroscopic pattern; for example, papillomas are benign epithelial tumors with certain growth characteristics, such as exophytic or finger-like projections.
10.1.1.2 Malignant Tumors
The classification of malignant tumors essentially follows that of benign tumors, with some exceptions. Malignant neoplasms arising from epithelial cells are termed carcinomas. Carcinomas are further classified on the basis of the type of epithelium, for example, glandular as adenocarcinoma (Fig. 10.2), squamous as squamous cell carcinoma (Fig. 10.3), and transitional as transitional cell carcinoma. Malignant epithelial tumors that have not extended through the underlying basement membrane are described as in situ carcinoma. Malignant tumors arising from mesenchymal tissue are broadly designated as sarcomas (Fig. 10.4). These are further subclassified on a histogenetic basis according to the normal tissue they resemble or its embryonal counterparts, for example, fibrosarcoma, chondrosarcoma, leiomyosarcoma, and rhabdomyosarcoma.
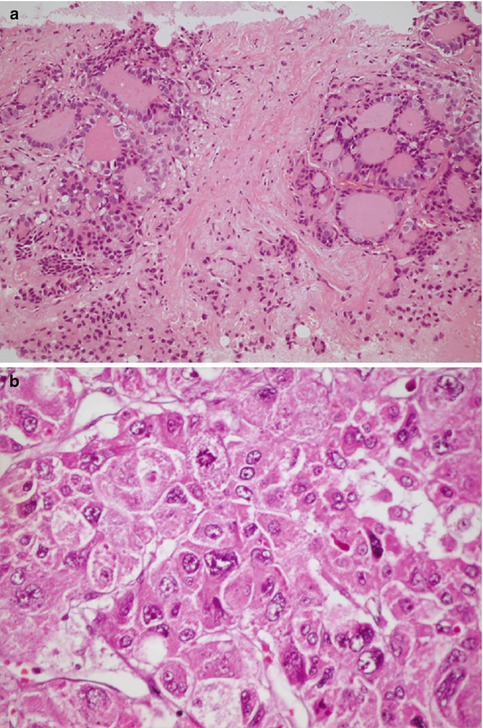
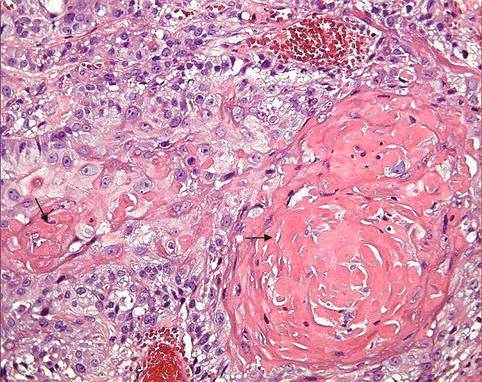
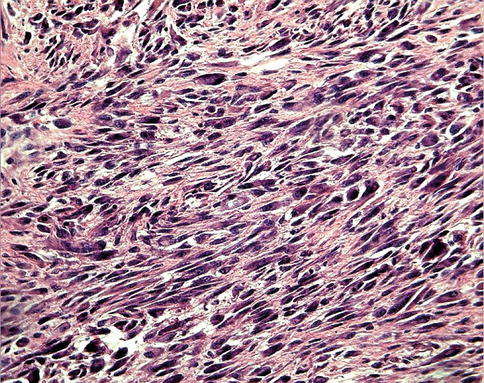

Fig. 10.2
Adenocarcinoma. (a) Example of adenocarcinoma in a case of metastatic thyroid cancer in the liver (H&E ×20) illustrating the glandular architacture. (b) A high power field of another adenocarcinoma (hepatocellular carcinoma) illustrating the typical cellular features of malignancy. Note the variation in cell size, abnormal chromatin pattern, prominent nucleoli, and abnormal mitosis (H&E ×40)

Fig. 10.3
Squamous cell carcinoma. The tumor consists of well-differentiated squamous cells forming keratin pearls (arrows)

Fig. 10.4
Soft tissue sarcoma consisting of pleomorphic spindle cells, illustrating the features of malignancy as variable cell size and shapes and increased nuclear/cytoplasmic ratio
There are tumors that do not follow any classification scheme, and they have been identified by trivial names, such as seminoma and melanoma. Other tumors carry eponyms, such as Hodgkin’s disease and Ewing’s sarcoma.
Tumors within a single organ or single type of epithelium are further subclassified into different types; each has its own characteristics, prognosis, and response to therapy. Malignant tumors that extend into surrounding tissue without respecting normal tissue boundaries, are capable of invading lymphatics and blood vessels, and can be transported to distant sites. Several salient abnormalities are helpful to the pathologist in making the morphological diagnosis of such tumors, and they are expressed in two ways: First, there are abnormalities that affect individual cells in the form of cytological features and increased mitotic activity. Cytological features of malignancy include cell enlargement, increased ratio of nuclear to cytoplasmic area, pleomorphism (variation in size and shape), clumping of nuclear chromatin, and big nucleoli (Fig. 10.2b). Second, there are abnormalities that affect intercellular relationship, i.e., altered orientation of neoplastic cells and stroma leading to disorganization [3].
10.1.2 Tumor Grading
Grading is a scheme that attempts to determine the degree of malignancy and is based on the evaluation of certain parameters that vary according to the system used. These broadly include degree of tumor cellularity, resemblance of tumor cells to their normal forbears morphologically and functionally, cellular pleomorphism or anaplasia, mitotic activity (number and abnormality), and necrosis [4]. Tumors may be graded on four-tier, three-tier, or two-tier scales, depending on the tumor type. In general, a three-grade system has proven to be the most reproducible: well, moderately, and poorly or undifferentiated, or grades I, II, and III, where grade 1 is well differentiated (low grade); grade 2, moderately differentiated (intermediate grade); and grade 3, poorly differentiated (high grade).
10.1.3 Tumor Staging
Staging of cancer depends on the size of the primary neoplasm, its extent to regional lymph nodes, and the presence or absence of metastasis. Cancer care is a cooperative, multidisciplinary endeavor; therefore, all disciplines involved in cancer care are to work well together, they must be able to communicate with precision. The TNM system has been developed by the American (American Joint Committee on Cancer, AJCC) and European (Union International Contre le Cancer, UICC) commissions on cancer to allow systematic categorization and description of cancer patients. In this fashion, disease progression patterns, natural history, and treatment outcome can be more reliably documented when applied to the individual patient.
The objectives of a staging system can be briefly summarized as follows: (a) to aid the clinician in planning treatment, (b) to give some indication of prognosis, (c) to assist the evaluation of end results, (d) to facilitate the exchange of information between treatment centers, and (e) to assist in the continuing investigation of cancer. The TNM system meets these requirements. It is an expression of the anatomical extent of disease and is based on the assessment of three components:
T
The extent of primary tumor
N
The absence or presence and extent of regional lymph node metastases
M
The absence or presence of distant metastases
TNM clinical-diagnostic staging allows for pretreatment characterization via clinical examination and specific diagnostic studies. TNM surgical-evaluative staging is applied following a major surgical exploration or biopsy. TNM postsurgical treatment-pathological staging characterizes the extent of the cancer following thorough examination of the resected surgical specimen. TNM retreatment staging is applied in instances where the initial therapy has failed and additional treatment decisions are being considered. TNM autopsy staging is the final staging, done after the postmortem study.
10.1.4 Tumor Growth Rate
Generally, most benign neoplasms grow slowly, and most malignant neoplasms grow much faster. However, there are many exceptions since some benign neoplasm grow more rapidly than some cancers. For example, uterine leiomyomas (benign smooth muscle tumors) may increase rapidly in size during pregnancy (hormonal effect). The rate of growth of malignant tumors correlates in general with their level of differentiation. Rapidly growing malignant neoplasm often contains central areas of ischemic necrosis because the tumor blood supply, derived from the host, fails to keep pace with the oxygen needs of the expanding mass of cells [1].
10.2 Tumor Biology
Developing cancer cells must acquire a variety of characteristics not generally found in non-transformed cells. The cancer cell represents the culmination of a complex process of developing capacity for largely unregulated growth.
The main characteristics that differentiate the cancer cell from the precancerous or noncancerous cell are capabilities of self-sufficiency in growth signals, insensitivity to antigrowth signals, evasion of apoptosis, limitless replicative potential, sustained angiogenesis, and a potential for tissue invasion and metastasis. In addition, the enabling characteristic of genetic instability was noted as a driving force for acquiring these cell characteristics.
10.2.1 Cell Growth and Cell Cycle
Normal cell proliferation (also known as cell growth or cell division) is essential for tissue homeostasis in the adult body. When stimulated to divide, a normal cell progresses through a tightly regulated process known as the cell cycle.
The cell cycle is a complex circuit composed of positive and negative protein regulators, the role of which is to duplicate DNA specifically during S phase and to segregate it evenly into two identical progeny during M phase (see Fig 2.3 Chap. 2). When a cell leaves the dormant state of G0 and enters a metabolically active phase during G1, the destiny of the cell cycle pivots in the equilibrium as the decision to undergo division must be made at the restriction point [5]. Because G1 is such an essential phase of the cell cycle, it is not astonishing that many oncogenic perturbations have been found as targeted amplifications or mutations of G1-specific protein regulators.
10.2.2 Tumor Neovascularization (Angiogenesis)
Cell survival and proliferation are dependent on an adequate supply of oxygen and nutrients and the removal of toxic metabolites. Tumor cells still require nutrients and oxygen in order to grow. Since oxygen can diffuse radially from capillaries for only 150–200 μm, the growth of tumor masses greater than 1 mm in diameter depends on the formation of new blood vessels, also known as tumor neovascularization or angiogenesis.
Neovascularization is a feature of neoplasia. Neovascularization supplies nutrients and oxygen and is required not only for continued tumor growth but also for metastasis [1].
Tumor-associated angiogenic factors may be produced by tumor cells or may be derived from inflammatory cells (e.g., macrophages) that infiltrate tumors. The two most important tumor-associated angiogenic factors are vascular endothelial growth factor (VEGF) and basic fibroblast growth factor. The tumor cells not only produce angiogenic factors but also induce antiangiogenesis molecules. The molecular basis of the angiogenesis is not entirely clear but may involve increased production of angiogenic factors or loss of angiogenesis inhibitors. Hypoxia within the growing tumor favors angiogenesis by release of hypoxia-inducible factor-1 (HIF-1) [1]. HIF-1 controls transcription of VEGF. The wild-type TP53 gene seems to inhibit angiogenesis by inducing the synthesis of the antiangiogenic molecule thrombospondin-1. With mutational inactivation of both TP53 alleles (a common event in many cancers), the levels of thrombospondin-1 drop precipitously, tilting the balance in favor of angiogenic factors.
Because of the important role of angiogenesis in tumor growth, extensive publication concentrated on antiangiogenesis therapy and the results are very promising.
10.2.3 Distinguishing Features of Tumor Cells
Cancer is a genetic disease resulting from multiple, sequential genetic changes affecting oncogenes, tumor suppressor genes, and modifiers [6]. Because of this multistep process, most human malignancies show various degrees of genetic heterogeneity even if they originate from single cells.
The multistep progression model determines that cells pass through a number of distinctive intermediate stages of evolution from normalcy to full malignancy [7]. The evolved tumor cells vary significantly from their normal counterparts.
Tumor cells have distinguishing morphological and structural features that are different from those of the cells of origin. Moreover, the abnormal cells show altered interaction with neighboring cells.
10.2.3.1 Loss of Contact Inhibition of Growth
Normal cells have an ordered growth pattern and a predicted relationship with their neighboring cells, and that growth pattern is predominantly two-dimensional. Further normal cell division is inhibited by contacts made with other cells; this is the phenomenon of contact inhibition [8]. In contrast, tumor cells exhibit loss of contact inhibition and continue to display a disordered growth pattern.
10.2.3.2 Growth Regulatory Pattern
Another feature that distinguishes normal growth from malignant proliferation is the reduced dependence of the latter on the presence of the known stimulatory and inhibitory growth factors. The diversion from the control of the growth regulatory factors can be explained, in part, by the discovery of biochemical changes within cancer cells resulting from some genetic alterations. Thus, when normal cells are grown in culture, they continue to divide for a limited number of generations and then experience a senescent crisis, in which most cells stop dividing and die and no cells survive to establish permanent cell lines. On the other hand, human tumor cells usually have an unlimited potential for growth and are thus immortalized [9].
This distinctive growth factor independence or autonomy has been attributed to several mechanisms including ability of tumor cells to secrete mitogenic growth factors that have a growth-stimulatory ability on the same cell that has released them [10].
10.2.3.3 Ability to Escape Immune Surveillance Pathways (Immune Evasion)
Another characteristic feature of human malignancy is its ability to escape the human immune surveillance pathways. It is known that human tumor cells express on their surfaces novel antigens that are not present on the surfaces of their untransformed progenitors. One type of novel antigen may be common to many different types of malignancy and may be recognized by the natural killer lymphocytes even without specific prior immunization [5]. In other instances, novel antigens specific to a particular type of tumor may be displayed. According to one theory of tumorigenesis, all individuals develop abundant transformed cells over the course of their lives, but most of these cells are recognized and destroyed by one or another module of the host’s immune mechanisms. Support for this theory stems from the observation of the substantial increase in the incidence of malignancy in patients with acquired immune deficiency syndrome, in patients with organ transplants receiving intensive immunosuppressive agents, and in patients with other immune deficiency disorders. It has been proven that tumor cells may escape the host immune system by downregulating the expression of HLA antigens, which normally assist lymphocyte recognition of the target cells.
10.2.3.4 Metabolic Alterations
Tumor cells exhibit a vast array of metabolic differences distinguishing them from their untransformed counterparts. This is illustrated primarily in simplified metabolic activities and by an increased synthesis of material necessary for cell division. Some of the most striking metabolic alterations include the utilization of anaerobic pathways and the increased utilization of glucose transport [11].
10.2.4 Invasion and Metastasis
Malignant neoplasms disseminate by one of three pathways: (1) seeding within body cavities—an example of this is carcinoma of the colon that may penetrate the wall of the gut and reimplant at other sites in the peritoneal cavity, (2) lymphatic spread which is the preferred way of spread by carcinomas in general, or (3) hematogenous spread which is favored by sarcomas. The ability of a tumor to metastasize is a multifaceted phenomenon which requires several prerequisites [12]: (a) invasion by tumor cells through adjacent structures, (b) entrance of tumor cells into blood or lymphatic vessels, (c) survival of tumor cells within the circulation and avoidance of the immune system, and (d) implantation in a foreign tissue with establishment of a new tumor locus.
Most carcinomas arise in epithelial cell layers with an underlying basement membrane. Invasive tumors frequently secrete enzymes, including collagenases, heparanase, and stromelysin, that are capable of degrading this type of physical barrier [13]. Once a tumor has eroded through the wall of a blood or lymphatic vessel, individual tumor cells may detach and circulate through the body as an embolus. Encasing these cells in clots of fibrin or in aggregates of platelets may protect them from destruction by the immune system. The presence of tumor cells in the circulation does not necessary lead to metastases. However, these circulating cells have to “home” preferentially to a specific target organ. This pattern has been described as the “seeds” and “soil” model and has been demonstrated experimentally and observed consistently in oncological practice. It is not clear how these patterns of metastases arise, but some evidence suggests that tumor cells can respond to specific chemotactic signals. Moreover, specific receptors have recently been identified on the surfaces of metastasizing tumor cells that cause them to adhere to complementary structures displayed by endothelial cells in certain organs [14].
10.2.5 Carcinogenesis
Carcinogenesis or oncogenesis is a process by which normal cells are transformed into cancer cells. It is well known that agents that induced damage of the DNA (mutagens) have potential carcinogenic effects. The progenitor tumor cells that sustain genetic alterations are said to undergo somatic mutations that promote the multistep process of neoplastic development.
10.2.5.1 Genetic Mutations and Cellular Oncogenes
Enormous scientific evidence has accumulated over more than half a century to indicate that neoplastic transformation occurs as a direct consequence of alterations to the cell genome.
Four basic approaches have been used to identify genes involved in cancer: (a) the study of cancer-causing viruses, (b) bioassays for cancer genes in tissue culture systems, (c) localization of genes at sites of chromosomal alteration in tumor specimens, and (d) isolation of genes for cancer-predisposing familial syndromes.
It is well known that agents that induce damage of the DNA (mutagens) have potential carcinogenic effects. The DNA found in the nucleus of every living cell encodes the heritable or genetic information necessary to direct the development of that organism from a single fertilized cell to the mature organism. Therefore, the progenitor tumor cells that sustain genetic alterations are said to undergo somatic mutations that promote the multistep process of neoplastic development.
From many studies, it became very clear that some of the information encoding cancerous behavior of the donor cells could be passed to the recipient cells via DNA molecules. It was not until the early 1980s that, with the advent of gene cloning, it was possible to isolate these cellular oncogenes (Table 10.1).
Table 10.1
Cellular oncogenes implicated in human cancer
Category | Proto-oncogene | Mode of activation | Associated human tumor |
|---|---|---|---|
Growth factors | |||
PDGF-β chain | S/S | Overexpression | Astrocytoma |
Osteosarcoma | |||
Fibroblast growth factors | HST–1 | Overexpression | Stomach cancer |
INT–2 | Amplification | Bladder cancer | |
Breast cancer | |||
Melanoma | |||
TGFa | TGFa | Overexpression | Astrocytomas |
Hepatocellular carcinomas | |||
HGF | HGF | Overexpression | Thyroid cancer |
Growth factor receptors | |||
EGF receptor family | ERB–B1 (ECFR) | Overexpression | Squamous cell carcinomas of lung, gliomas |
ERB–B2 | Breast and ovarian cancers | ||
CSF-1 receptor | FMS | Point mutation | Leukemia |
Receptor for neutrotrophic factors | RET | Point mutation | Multiple endocrine neoplasia 2A and B, familial medullary thyroid carcinomas |
PDGF receptor | PDGF–R | Overexpression | Gliomas |
Proteins involved in signal transduction | |||
GTP binding | K–RAS | Point mutation | Colon, lung, and pancreatic tumors |
H–RAS | Point mutation | Bladder and kidney tumors | |
N–RAS | Point mutation | Melanomas | |
Non-receptor tyrosine kinase | ABL | Translocation | Chronic myeloid leukemia |
WNT signal transduction | β-catenin | Point mutation | Hepatoblastomas, hepatocellular carcinoma |
Overexpression | |||
Nuclear regulatory proteins | |||
Transcriptional activators | C–MYC | Translocation | Burkitt’s lymphoma |
Cell cycle regulators | |||
Cyclins | CYCLIN D | Translocation | Mantle cell lymphoma |
Amplification | Breast and esophageal cancers | ||
CYCLIN E | Overexpression | Breast cancer | |
Cyclin-dependent kinase | CDK4 | Amplification or point mutation | Glioblastoma, melanoma |
Activation of cellular oncogenes is a complex process that involves a variety of somatic mutational mechanisms. The c-myc oncogene develops its malignant properties through mechanisms that affect the level of expression of its encoded proteins without any associated alteration in its protein structure. Recently, the prognostic significance of c-myc in lymphoma has also been described [15].
On the other hand, a quantitative change in the structure of the encoded proteins may be responsible for oncogene activation. In chronic myelogenous leukemia, for example, the abl gene undergoes fusion with a fully unrelated gene, bcr. The bcr-abl hybrid protein encoded by these fused genes differs substantially in structure and function from the normal abl proto-oncogene protein [16].
It was clear, however, that single oncogenes were not capable of inducing transformation of fully normal cells into totally malignant cells. Instead, the action of a single oncogene usually induces only partial progression to malignancy. Fortunately, this has served as a protective mechanism to prevent the development of cancers in response to single oncogenes that arise through isolated genetic mishaps. Therefore, it appears that each step through which cells pass in the progression from normalcy to malignancy (multistep carcinogenesis) is characterized by a distinct genetic change, often one that creates an oncogene. However, the precise number of the distinct steps for most human tumors is poorly understood.
Nonlethal genetic damage may be acquired by the action of environmental agents, such as chemicals, radiation, or viruses, or it may be inherited in the germ line. The current hypothesis implies that cancer formed as a result of clonal expansion of a single progenitor cell that has incurred the genetic damage (i.e., tumors are monoclonal). This theory has been supported by many studies that revealed clonality in neoplasm that have been assessed readily in women who are heterozygous for polymorphic X-linked markers, such as the enzyme glucose-6-phosphate dehydrogenase or X-linked restriction fragment length polymorphisms or in clonality assessed in lymphoid neoplasm. Mutant alleles of proto-oncogenes are called oncogenes [17, 18].
Three classes of normal regulatory genes—growth-promoting proto-oncogenes; growth-inhibiting cancer suppressor genes (antioncogenes); and genes that regulate programmed cell death, or apoptosis—are the principal targets of genetic damage [1]. They are considered dominant because they transform cells despite the presence of their normal counterpart. In addition to the three classes of genes mentioned earlier, a fourth category of genes, those that regulate repair of damaged DNA, is pertinent in carcinogenesis.
10.2.5.2 Growth-Promoting Proto-oncogenes
Genes that promote autonomous cell growth in cancer cells are called oncogenes. They are derived by mutations in proto-oncogenes and are characterized by the ability to promote cell growth in the absence of normal growth-promoting signals. Their products, called oncoproteins, resemble the normal products of proto-oncogenes except that oncoproteins are devoid of important regulatory elements, and their production in the transformed cells does not depend on growth factors or other external signals.
All normal cells require stimulation by growth factors to undergo proliferation. Many cancer cells acquire growth self-sufficiency, however, by acquiring the ability to synthesize the same growth factors to which they are responsive. Such is the case with platelet-derived growth factor (PDGF) and transforming growth factor α (TGF-α). Many glioblastomas secrete PDGF, and sarcomas make TGF-α.
10.2.5.3 Growth Factor Receptors
There are several oncogenes that encode growth factor. Those oncogenes represent either mutation or overexpression of normal forms of growth factor receptors. Mutant receptor proteins can send continuous mitogenic signals to cells, even in the absence of the growth factor in the environment [1]. Overexpression can render cancer cells hyperresponsive to normal levels of the growth factor, a level that would not normally trigger proliferation. Among the examples of overexpression is the receptor called HER2 (ERBB2), which is present in 30 % of breast cancers and present in variable percentages in other human cancer. Breast cancers which are positive for Her2 are more sensitive to the mitogenic effects of small amounts of growth factors, and a high level of HER2 protein in breast cancer is associated with poor prognosis. The clinical significance of HER2 in breast cancers is clearly evident by the treatment of breast cancer with anti-HER2 antibodies which block the extracellular domain of this receptor [19, 20].
10.2.5.4 Signal-Transducing Proteins
Mutations in genes that encode various components of the signaling pathways are a common process in cancer. These signaling molecules couple growth factor receptors to their nuclear targets. The most important members in this category are RAS and ABL.
Approximately 30 % of all human tumors contain mutated versions of the RAS gene. In some tumors, such as colon and pancreatic cancers, the incidence of RAS mutations is even higher [1]. The activated RAS in turn activates downstream regulators of proliferation, including the RAF-MAP kinase mitogenic cascade, which flood the nucleus with signals for cell proliferation. In chronic myeloid leukemia and certain acute leukemias, this activity is unleashed because the ABL gene is translocated from its normal abode on chromosome 9 to chromosome 22, where it fuses with part of the breakpoint cluster region (BCR) gene. The BCR-ABL hybrid gene has potent tyrosine kinase activity, and it activates several pathways, including the RAS-RAF cascade just described. The crucial role of BCR-ABL in transformation has been confirmed by the dramatic clinical response of patients with chronic myeloid leukemia after therapy with an inhibitor of ABL kinase called STI 571 (Gleevec); this is another example of rational drug design emerging from an understanding of the molecular basis of cancer.
10.2.5.5 Nuclear Transcription Factors
Growth autonomy in neoplasm may occur as a consequence of mutations affecting genes that regulate transcription of DNA such as MYC oncogene that has been localized to the nucleus. The MYC proto-oncogene is expressed in virtually all cells, and the MYC protein is induced rapidly when quiescent cells receive a signal to divide. The MYC protein binds to the DNA, causing transcriptional activation of several growth-related genes, including cyclin-dependent kinases (CDKs), whose product drives cells into the cell cycle. In normal cells, MYC levels decline to near basal level when the cell cycle begins. In contrast, oncogenic versions of the MYC gene are associated with persistent expression or overexpression, contributing to sustained proliferation. The classic example is the dysregulation of the MYC gene resulting from a t(8; 14) translocation that occurs in Burkitt’s lymphoma [1].
10.2.5.6 Tumor Suppressor Genes and Tumor Progression
While oncogenes play a critical role in tumorigenesis, another group of genes known as tumor suppressors appears to be equally significant. These tumor suppressor genes, as the name implies, function in the normal cells to restrict cellular proliferation. However, tumor suppressor genes are involved in tumorigenesis when they suffer genetic inactivation or loss-of-function mutations, affecting the two redundant copies of these genes and resulting in the elimination of that important barrier to cell growth.
The loss of wild-type tumor suppressor genes such as Rb and p53 is associated with a wide variety of human tumors. Many proto-oncogenes transform in model systems in which the wild-type gene is overexpressed or expressed in the wrong cell type; similarly, overexpression or inappropriate expression of some proto-oncogenes, such as c-myc, is thought to be tumorigenic in some human tissues.
The most commonly mutated tumor suppressor gene in human cancer is p53, with at least 50 % of tumors having abnormal p53 genes [21]. The gene participates in a cell cycle checkpoint signal transduction pathway that causes either a G1 arrest or apoptotic cell death following DNA damage. Loss of p53 function during tumorigenesis can thus result in both inappropriate progressions through the cell cycle after DNA damage and survival of a cell that might otherwise have been destined to die. Another newly discovered tumor suppressor gene is the p16. Since its discovery as a CDKI (cyclin-dependent kinase inhibitor) in 1993, the tumor suppressor p16 has gained widespread importance in tumor biology [22]. The frequent mutations and deletions of p16 in human cancer cell lines first suggested an important role for p16 in carcinogenesis.
APC is another tumor suppressor gene [23] and its loss is common in colon cancer. APC is a cytoplasmic protein whose function in normal cells is to bind another protein called β-catenin and bring about its degradation. β-Catenin is a transcriptional factor, and, if APC function is defective by mutation, accumulated level of β-catenin occurs in the cells, driving cell proliferation. Individuals born with one mutant allele develop hundreds to thousands of adenomatous polyps in the colon during their teens or 20s. If the cells develop a second mutation of the normal inherited gene on the other allele, it leads to development of carcinoma of the colon.
10.2.6 Apoptosis
Each day, approximately 50–70 billion cells die in the average adult because of programmed cell death. The morphological ritual cells go through when experiencing programmed cell death has been termed apoptosis and is executed by a family of intracellular proteases, called caspases [24]. These physiological deaths culminate in fragmentation of cells into membrane-encased bodies, which are cleared through phagocytosis, by neighboring cells without inciting inflammatory reactions or tissue scarring. Defects in the processes controlling apoptosis can extend cell life span, contributing to neoplastic cell expansion independent of cell division [25].
10.2.7 Hereditary Cancer
The evidence now indicates that for many types of cancer, including the most common forms, there exist not only environmental influences but also hereditary predispositions. Our list of genes whose mutations can account for hereditary cancer is increasing. Hereditary forms of cancer can be divided into three categories:
10.2.7.1 Inherited Cancer Syndromes
Inherited cancer syndromes include several well-defined cancers in which inheritance of a single mutant gene greatly increases the risk of a person developing a tumor. The predisposition to these tumors shows an autosomal dominant pattern of inheritance. Childhood retinoblastoma is the most striking example of this category.
Familial adenomatous polyposis is the other classic example. For most the development of the clinical features is age dependent, and that development may be early in life, as with hereditary retinoblastoma, or relatively later in life as with colorectal carcinoma.
10.2.7.2 Familial Cancers
Almost all the common types of cancers that occur sporadically have been reported to occur in familial forms. Examples include carcinomas of the colon and breast. Inherited susceptibility to breast cancer has been an area of intensive investigation for the past 10 years. Early work focused on identifying modes of transmission, which culminated in the identification of chromosome 17q12–21 as the first human genomic region that harbored an autosomal dominant susceptibility gene for breast cancer (BRCA1) in 1990. BRCA1 was subsequently identified, followed shortly by the identification of BRCA2 [26]. Research has elucidated much about the mutation spectrum and mutation frequency of these genes in specific populations in the past 3 years and is beginning to identify potential functions. Whereas progress in this area has been rapid and much is now known about inherited susceptibility to breast cancer, much more needs to be done to make these discoveries useful in the diagnosis, treatment, and ultimately prevention of breast cancer.
10.2.7.3 Autosomal Recessive Syndromes of Defective DNA Repair
One of the best-studied examples is xeroderma pigmentosum, in which DNA repair is defective. It is a rare autosomal recessive disease characterized by deficiency of endonuclease, the enzyme partly responsible for repair of DNA damage. Children with this disorder develop multiple squamous cell carcinomas [18].
10.3 Tumor Imaging and Pathophysiological Correlation
Understanding tumor biology is crucial for the use of radionuclide agents for tumor imaging and therapy. For example, the features of proliferation, apoptosis, necrosis, angiogenesis, and cell receptors lead to the discovery of newer radiopharmaceuticals and direct the use of the various radiopharmaceuticals for imaging and treatment of tumors based on the biological features. The use of positron-emitting radionuclide imaging has revolutionized the imaging of physiologically and pathologically important molecules. It provides data at both a molecular and metabolic level needed for the evaluation and treatment for effective patient management. It has a major role in cancer detection and more importantly in staging and follow-up of many tumors (Table 10.2). In the view of lack of PET, particularly in developing countries, utilizing non-PET radiotracers in managing tumors and in particular in monitoring therapeutic effects of chemoradiotherapy is feasible. Accordingly a brief discussion of such radiopharmaceuticals and their applications is included in this chapter.
Table 10.2
Major uses of PET/CT in oncology
Diagnosis | Head and neck cancer |
Lung cancer | |
Pancreatic cancer | |
Unknown primary tumor | |
Staging | Breast cancer |
Colorectal cancer | |
Esophageal cancer | |
Head and neck cancer
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|