Fig. 1.
From left to right: LA region definition; surface projection to SUM; Radial sampling of the four quadrants around each PV; Representation in polar coordinates (only one vein in this case).
2.2 Scar Segmentation
The classification of voxel intensities of the LA wall into scar and healthy tissue was done using the unsupervised clustering method based on normalized intensity [9]. This algorithm is implemented in the module “Automatic Left Atrial Scar” of the Cardiac MRI Toolkit [10] for 3D Slicer [11, 12]. The output of the algorithm is a binary mask including LA wall points where scar tissue has been detected. The segmented scar tissue was then projected onto the LA endocardial surface mesh (built using Marching Cubes [13]) by sampling the mask labels along the normals to the wall.
2.3 Standardized Unfold Map (SUM) of the Left Atrium
The scar information obtained in the previous step (Sect. 2.2) is represented as a 2-manifold embedded in 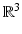 (scar on LA endocardial surface mesh), where it is not straightforward to analyze and compare data. In order to facilitate the analysis of scar information for gap quantification we represent the atrium in a flat domain using the Standardized Unfold Map (SUM) of the LA [7]. The SUM representation is a disk divided into 24 left atrial regions and with a number of holes corresponding to the pulmonary veins, as it is illustrated in Fig. 1. The SUM is calculated by a quasi-conformal flattening of the LA surface mesh under the constraints that the mitral annulus is mapped to the exterior boundary of a disk, while the PV and the LA appendage are mapped to predefined holes within that disk. The position and size of these holes are defined as to facilitate the analysis in accordance with clinical requirements. The consistency of mappings from different patients is handled through the currents-based surface registration technique recently proposed by Durrleman et al. [14], which is implemented in the freely available Open-Source software Deformetrica [15]. The whole pipeline to generate a SUM for the LA can be summarized by the following steps, given an already defined template atrium (e.g. an average atrium) and its flattening (calculated here using Graphite [16]): (a) The PV of the LA surface are automatically clipped at 10 mm from the ostia to ensure the same length of the PV trunks in all LA anatomies. The method requires manual selection of four seed points corresponding to each PV; (b) currents-based surface registration of every atrium to the template atrium; (c) Closest point mapping of scar information (scar binary mask) from the analyzed atrium to the template atrium and to its flattened representation.
(scar on LA endocardial surface mesh), where it is not straightforward to analyze and compare data. In order to facilitate the analysis of scar information for gap quantification we represent the atrium in a flat domain using the Standardized Unfold Map (SUM) of the LA [7]. The SUM representation is a disk divided into 24 left atrial regions and with a number of holes corresponding to the pulmonary veins, as it is illustrated in Fig. 1. The SUM is calculated by a quasi-conformal flattening of the LA surface mesh under the constraints that the mitral annulus is mapped to the exterior boundary of a disk, while the PV and the LA appendage are mapped to predefined holes within that disk. The position and size of these holes are defined as to facilitate the analysis in accordance with clinical requirements. The consistency of mappings from different patients is handled through the currents-based surface registration technique recently proposed by Durrleman et al. [14], which is implemented in the freely available Open-Source software Deformetrica [15]. The whole pipeline to generate a SUM for the LA can be summarized by the following steps, given an already defined template atrium (e.g. an average atrium) and its flattening (calculated here using Graphite [16]): (a) The PV of the LA surface are automatically clipped at 10 mm from the ostia to ensure the same length of the PV trunks in all LA anatomies. The method requires manual selection of four seed points corresponding to each PV; (b) currents-based surface registration of every atrium to the template atrium; (c) Closest point mapping of scar information (scar binary mask) from the analyzed atrium to the template atrium and to its flattened representation.
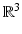 (scar on LA endocardial surface mesh), where it is not straightforward to analyze and compare data. In order to facilitate the analysis of scar information for gap quantification we represent the atrium in a flat domain using the Standardized Unfold Map (SUM) of the LA [7]. The SUM representation is a disk divided into 24 left atrial regions and with a number of holes corresponding to the pulmonary veins, as it is illustrated in Fig. 1. The SUM is calculated by a quasi-conformal flattening of the LA surface mesh under the constraints that the mitral annulus is mapped to the exterior boundary of a disk, while the PV and the LA appendage are mapped to predefined holes within that disk. The position and size of these holes are defined as to facilitate the analysis in accordance with clinical requirements. The consistency of mappings from different patients is handled through the currents-based surface registration technique recently proposed by Durrleman et al. [14], which is implemented in the freely available Open-Source software Deformetrica [15]. The whole pipeline to generate a SUM for the LA can be summarized by the following steps, given an already defined template atrium (e.g. an average atrium) and its flattening (calculated here using Graphite [16]): (a) The PV of the LA surface are automatically clipped at 10 mm from the ostia to ensure the same length of the PV trunks in all LA anatomies. The method requires manual selection of four seed points corresponding to each PV; (b) currents-based surface registration of every atrium to the template atrium; (c) Closest point mapping of scar information (scar binary mask) from the analyzed atrium to the template atrium and to its flattened representation.
(scar on LA endocardial surface mesh), where it is not straightforward to analyze and compare data. In order to facilitate the analysis of scar information for gap quantification we represent the atrium in a flat domain using the Standardized Unfold Map (SUM) of the LA [7]. The SUM representation is a disk divided into 24 left atrial regions and with a number of holes corresponding to the pulmonary veins, as it is illustrated in Fig. 1. The SUM is calculated by a quasi-conformal flattening of the LA surface mesh under the constraints that the mitral annulus is mapped to the exterior boundary of a disk, while the PV and the LA appendage are mapped to predefined holes within that disk. The position and size of these holes are defined as to facilitate the analysis in accordance with clinical requirements. The consistency of mappings from different patients is handled through the currents-based surface registration technique recently proposed by Durrleman et al. [14], which is implemented in the freely available Open-Source software Deformetrica [15]. The whole pipeline to generate a SUM for the LA can be summarized by the following steps, given an already defined template atrium (e.g. an average atrium) and its flattening (calculated here using Graphite [16]): (a) The PV of the LA surface are automatically clipped at 10 mm from the ostia to ensure the same length of the PV trunks in all LA anatomies. The method requires manual selection of four seed points corresponding to each PV; (b) currents-based surface registration of every atrium to the template atrium; (c) Closest point mapping of scar information (scar binary mask) from the analyzed atrium to the template atrium and to its flattened representation.2.4 Gap Quantification
In the SUM, the anatomical regions around each PV are represented in four quadrants (see Fig. 1). An ideal ablation lesion isolating the PV from the rest of the atrium is expected to be a connected scar surrounding the PV. However the scar is not always connected and some gaps of healthy tissue may appear. We propose to transform the surroundings of every PV into a rectangular domain using a polar coordinate transformation centered at each PV hole in the SUM, as shown in Fig. 1. An eventual continuous ablation lesion running from left to right in this rectangular map would correspond to a successful ablation completely isolating PV from the rest of the atria.
To construct the rectangular map every quadrant around each PV is radially sampled using a set of rays connecting the centre of the PV with the boundaries of the quadrant at radial angle intervals of 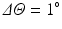 producing 90 rays per quadrant. We represent the radial angles along the horizontal axis, and the distance from the PV origin along the vertical axis. Since PV regions are not circular, ray segments will have different lengths. In order to have a convenient representation (rectangle) we use the same number of samples (100) along every ray irrespectively of its length. Once the scar information around each PV is mapped onto the rectangular domain, these maps are post-processed using morphological opening and closing to eliminate the possible small noisy structures. Several examples of these rectangular maps are shown in Fig. 2.
producing 90 rays per quadrant. We represent the radial angles along the horizontal axis, and the distance from the PV origin along the vertical axis. Since PV regions are not circular, ray segments will have different lengths. In order to have a convenient representation (rectangle) we use the same number of samples (100) along every ray irrespectively of its length. Once the scar information around each PV is mapped onto the rectangular domain, these maps are post-processed using morphological opening and closing to eliminate the possible small noisy structures. Several examples of these rectangular maps are shown in Fig. 2.
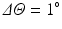 producing 90 rays per quadrant. We represent the radial angles along the horizontal axis, and the distance from the PV origin along the vertical axis. Since PV regions are not circular, ray segments will have different lengths. In order to have a convenient representation (rectangle) we use the same number of samples (100) along every ray irrespectively of its length. Once the scar information around each PV is mapped onto the rectangular domain, these maps are post-processed using morphological opening and closing to eliminate the possible small noisy structures. Several examples of these rectangular maps are shown in Fig. 2.
producing 90 rays per quadrant. We represent the radial angles along the horizontal axis, and the distance from the PV origin along the vertical axis. Since PV regions are not circular, ray segments will have different lengths. In order to have a convenient representation (rectangle) we use the same number of samples (100) along every ray irrespectively of its length. Once the scar information around each PV is mapped onto the rectangular domain, these maps are post-processed using morphological opening and closing to eliminate the possible small noisy structures. Several examples of these rectangular maps are shown in Fig. 2.
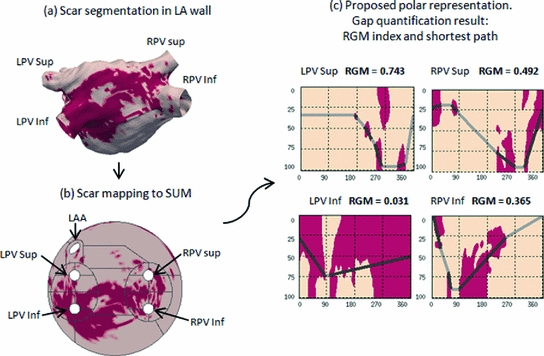
Fig. 2.
Pipeline of the method. (a) Scar segmentation in the LA wall mapped to the LA surface. Dark color denotes tissue classified as scar. (b) Scar mapped to the SUM. (c) Visualization of the scar in the polar representation. The neighborhood of each PV is shown separately. The lines in (c) show the shortest isolating path and the RGM for each PV are shown on top of every subplot.
The identification and quantification of gaps is carried out in the rectangular map by estimating the shortest path that isolates the PV going through as little healthy tissue as possible starting from the left (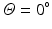 ) and finishing at the right side (
) and finishing at the right side (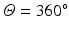 ). To this end a graph is constructed where each node represents every connected patch of scar in the rectangular map. The pairwise distances between all patches, computed using the distance transform, are associated to graph edges. Two auxiliary nodes on the horizontal extremes of the rectangular map (left and right sides) are added. Finally the Dijkstra algorithm [17] is used to estimate the shortest isolating path connecting these auxiliary nodes.
). To this end a graph is constructed where each node represents every connected patch of scar in the rectangular map. The pairwise distances between all patches, computed using the distance transform, are associated to graph edges. Two auxiliary nodes on the horizontal extremes of the rectangular map (left and right sides) are added. Finally the Dijkstra algorithm [17] is used to estimate the shortest isolating path connecting these auxiliary nodes.
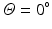 ) and finishing at the right side (
) and finishing at the right side (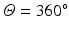 ). To this end a graph is constructed where each node represents every connected patch of scar in the rectangular map. The pairwise distances between all patches, computed using the distance transform, are associated to graph edges. Two auxiliary nodes on the horizontal extremes of the rectangular map (left and right sides) are added. Finally the Dijkstra algorithm [17] is used to estimate the shortest isolating path connecting these auxiliary nodes.
). To this end a graph is constructed where each node represents every connected patch of scar in the rectangular map. The pairwise distances between all patches, computed using the distance transform, are associated to graph edges. Two auxiliary nodes on the horizontal extremes of the rectangular map (left and right sides) are added. Finally the Dijkstra algorithm [17] is used to estimate the shortest isolating path connecting these auxiliary nodes.As a measure of PV isolation we propose to use the total length of healthy tissue crossed along the shortest isolating path, normalized by the total length of the path. Therefore, we define the Relative Gap Measure (RGM) as: 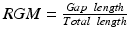 . Values of RGM equal to 0 mean that there are no gaps along the shortest isolating path and the PV is then electrically isolated. Any non-zero value of RGM would represent the presence of a gap around the studied PV, larger values of RGM corresponding to more gaps between scar patches. Figure 2 shows the rectangular maps corresponding to four pulmonary veins of a left atria together with the estimated shortest isolating paths and their associated RGM values. One can see two distinct situations in these atria: while the left inferior PV (LPV Inf) is almost completely surrounded by scar tissue, which is represented with a RGM value of 0.031, the left superior PV (LPV Sup) is surrounded by healthy tissue, which is characterized by a RGM value equal to 0.743. Therefore we can say that the LPV Inf is quite well electrically isolated while the LPV Sup has a big amount of gap.
. Values of RGM equal to 0 mean that there are no gaps along the shortest isolating path and the PV is then electrically isolated. Any non-zero value of RGM would represent the presence of a gap around the studied PV, larger values of RGM corresponding to more gaps between scar patches. Figure 2 shows the rectangular maps corresponding to four pulmonary veins of a left atria together with the estimated shortest isolating paths and their associated RGM values. One can see two distinct situations in these atria: while the left inferior PV (LPV Inf) is almost completely surrounded by scar tissue, which is represented with a RGM value of 0.031, the left superior PV (LPV Sup) is surrounded by healthy tissue, which is characterized by a RGM value equal to 0.743. Therefore we can say that the LPV Inf is quite well electrically isolated while the LPV Sup has a big amount of gap.
 . Values of RGM equal to 0 mean that there are no gaps along the shortest isolating path and the PV is then electrically isolated. Any non-zero value of RGM would represent the presence of a gap around the studied PV, larger values of RGM corresponding to more gaps between scar patches. Figure 2 shows the rectangular maps corresponding to four pulmonary veins of a left atria together with the estimated shortest isolating paths and their associated RGM values. One can see two distinct situations in these atria: while the left inferior PV (LPV Inf) is almost completely surrounded by scar tissue, which is represented with a RGM value of 0.031, the left superior PV (LPV Sup) is surrounded by healthy tissue, which is characterized by a RGM value equal to 0.743. Therefore we can say that the LPV Inf is quite well electrically isolated while the LPV Sup has a big amount of gap.
. Values of RGM equal to 0 mean that there are no gaps along the shortest isolating path and the PV is then electrically isolated. Any non-zero value of RGM would represent the presence of a gap around the studied PV, larger values of RGM corresponding to more gaps between scar patches. Figure 2 shows the rectangular maps corresponding to four pulmonary veins of a left atria together with the estimated shortest isolating paths and their associated RGM values. One can see two distinct situations in these atria: while the left inferior PV (LPV Inf) is almost completely surrounded by scar tissue, which is represented with a RGM value of 0.031, the left superior PV (LPV Sup) is surrounded by healthy tissue, which is characterized by a RGM value equal to 0.743. Therefore we can say that the LPV Inf is quite well electrically isolated while the LPV Sup has a big amount of gap.Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree



