Key Facts
- •
Osteomalacia resulting from the presence of a bone or soft tissue lesion or tumor is known as oncogenic osteomalacia or tumor-induced osteomalacia (TIO).
- •
Adults are usually affected with nonspecific complaints such as bone and muscle pain, weakness, fatigue, and recurrent fractures.
- •
Hypophosphatemia, hyperphosphaturia, low 1,25-dihydroxy–vitamin D, normal serum calcium, and normal PTH levels are noted.
- •
Radiographs are the primary imaging method for identifying osteomalacia.
- •
Additional studies may be necessary to identify the causative, lesion.
- •
Prompt remission of symptoms and biochemical abnormalities follow successful lesion excision.
Osteomalacia, a common metabolic disease, has multiple familiar and a few less well-appreciated etiologies. One of the latter, known as oncogenic, paraneoplastic, oncogenous, or tumor-induced osteomalacia (TIO), is regarded as relatively rare with fewer than 150 reported cases. It has been clinically underestimated as a distinct entity since it shares osteomalacia, a nonspecific endpoint, with other commoner etiologies. McCance first considered a coexistent bone lesion as a cause of TIO. Hauge then suspected, and Prader proved, the link between a tumor and osteomalacia. To date, understanding of the pathogenesis and mechanisms associated with TIO is still evolving.
This review aims to provide evidence to medical generalists as well as specialists for considering TIO as a possible cause when classic etiologies for osteomalacia are absent. Biochemical, physiologic, genetic, and imaging criteria will be summarized to enable more fruitful investigations leading to recognition and documentation of TIO in both children and adults.
CLINICAL FEATURES
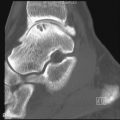
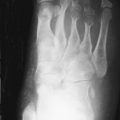
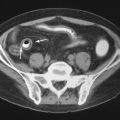
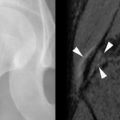
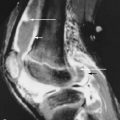
TIO has a male-to-female ratio of 1.2:1 and affects individuals aged 5 to 75 years. It usually occurs in adults over age 30 3 with nonspecific complaints such as bone and muscle pain, weakness, fatigue, and recurrent fractures, often attributed to osteoporosis. Children may present with gait disturbances, growth retardation, modeling deformities, and abnormal physes that, when recognized as rickets, lack a confirmed cause ( Box 36-1 ). Symptoms may be delayed, gradual, or precede the awareness of osteomalacia. Conversely, when osteomalacia is recognized, coexistent lesions may not be held responsible because most are familiar as independent entities. As a result, TIO has remained clinically elusive for several months to 20 years. Moreover, even when a coexistent lesion is considered, TIO remains a presumptive diagnosis until removal of the lesion is followed by prompt remission of all symptoms and biochemical aberrations. This healing may occur within hours to days ( Figure 36-1 ).
Bone and muscle pain
Weakness
Fatigue
Recurrent fractures
Gait disturbances
Growth retardation
Bony modeling deformities on radiographs
Rickets on radiographs



Lesions Responsible for TIO
Responsible benign or malignant primary and non-neoplastic TIO lesions occur in bones or soft tissues with approximately equal incidence. The most frequent bone sites are long bones, that is, femora or tibias. The next most frequent loci are in the craniofacial bones. Similar anatomic distributions are noted for the 50% of TIO lesions involving soft tissues.
Although diverse and unrelated, 70% to 80% of TIO lesions have been described as mesenchymal. Others histologically characterize 40% as “vascular,” with hemangiopericytoma representing 50% of vascular and 20% of all TIO lesions.
Hemangiopericytoma causes 20% of all cases of TIO.
Another large group described as “fibrohistiocytic and giant-cell lesions” contain prominent vasculature with less frequent osseous, cartilaginous, and neurologic components, while a third, with no clear-cut characteristics have been termed “mixed fibrovascular or mesenchymal.”
For convenience, known causative lesions have been grouped according to their predominant histologic features ( Box 36-2 ). While most often these lesions occur independently, all have been associated with the TIO syndrome. Nevertheless, none differ histologically, ultrastructurally, or immunohistochemically from their independent counterparts that have no TIO association. Most are small (0.5 to 15 cm), may grow slowly, or present prior to clinical complaints or establishment of osteomalacia. Consequently, coexistent biochemical and metabolic stigmata in general and osteomalacia in particular must be considered to determine their role.
GROUP 1 a VASCULAR
Hemangiopericytoma
Hemangioma
Hemangioendothelioma
Angiosarcoma
GROUP 2 b FIBROCYTIC
Nonossifying fibroma
Malignant fibrous histiocytoma
Neurofibromatosis
Polyostotic fibrous dysplasia
Fibrous xanthoma
GROUP 3 b GIANT CELL
Bone
Primary giant cell tumor
Giant-cell reparative granuloma
Giant-cell rich malignant fibrous histiocytoma
Soft tissue
Pigmented villonodular synovitis
GROUP 4 Bone/Cartilage/EPITHELIAL
Osteoblastoma
Osteosarcoma
Chondroma
Mesenchymal chondrosarcoma
Breast carcinoma
Prostatic carcinoma
GROUP 5 UNCLEAR CLASSIFICATION
“Vascular” fibrous histiocytoma
Malignant mesenchymal tumors
Undifferentiated sarcomas
a Group 1 most common.
b Groups 2 and 3 are the next most common.
BIOCHEMICAL AND PATHOPHYSIOLOGIC ASPECTS OF TIO
Phosphorus homeostasis is normally maintained by intestinal absorption interacting with intracellular and bone storage pools. It is vital for normal skeletal development and mineralization but is principally regulated by the kidney. Hypophosphatemia normally stimulates phosphorus proximal renal tubular reabsorption, calcitriol synthesis via 25-hydroxy–vitamin D-1 alpha-hydroxylase, with intestinal calcium and phosphorus absorption and mobilization from bone. However, the classic PTH-vitamin D axis fails to explain all facets of phosphorus homeostasis. While PTH does help regulate phosphorus reabsorption, it chiefly maintains calcium homeostasis, with recent investigators continuing to identify additional important regulators.
The biochemical aberrations of TIO are caused by phosphorus wasting and abnormal vitamin D metabolism.
TIO is manifested chemically by hypophosphatemia, hyperphosphaturia, low calcitriol (1,25-dihydroxy–vitamin D), normal serum calcium, and normal PTH levels.
Phosphorus wasting is caused by the inhibition of renal proximal tubular phosphorus reabsorption. Abnormal vitamin D metabolism prevents the compensatory rise in calcitriol (1,25-dihydroxy–vitamin D) normally stimulated by hypophosphatemia due to inhibition of the 1-alpha–hydroxylase enzyme by humoral factors.
TIO is diagnosed by identifying a lesion producing such a factor with resultant typical biochemical abnormalities ( Table 36-1 ) ( Box 36-3 ) with remission of the syndrome after its complete removal. However, diagnosis may be hampered or delayed since serum phosphorus determinations are not routinely included in standard laboratory metabolic profiles and must be specifically requested. Other conditions simulating TIO must also be excluded.
| Condition | Serum Phosphate | Urinary Phosphate | Serum Calcium | Urine Calcium | PTH | Di OH Vitamin D | Other |
|---|---|---|---|---|---|---|---|
| TIO | Low a | High b | Normal | Normal | Low c | ||
| XLHR | Low | High | Normal | Normal | Low | Children, osteosclerosis, calcified entheses Infrequent fractures, no muscle weakness | |
| ADHR | Low | High | Normal | Normal | Low | Children | |
| Hereditary hypophosphatemic rickets | Increased | Elevated |
a Due to decreased proximal tubular reabsorption.
b Due to increased P excretion.
- 1
Biochemical Screening
Serum P, Ca+, alkaline phosphatase, Creatinine, Intact PTH, 1,25-dihydroxy–vitamin D (calciferol), Fasting P, Urine Fasting 2 hr P, Creatinine, Ca, Amino acid, Glucose
- 2
Previously normal serum P
- 3
No family history of bone or mineral disorder, such as inherited hypophosphatemic rickets
- 4
Adult-onset progressive pain, weakness, multiple fractures without trauma
- 5
Symptoms more severe than other renal-phosphate wasting syndromes
- 6
Gene mutations—defective
PHEX: P-regulating gene—homologies to endopeptidases on X chromosome as in XLHR–X-linked hypophosphatemic rickets
FGF-23—Autosomal dominant hypophosphatemic rickets—ADHR; XLHR and ADHR both have family history, short stature, may have adult onset
NPT2—Major Na-P cotransporters in proximal renal tubule; hypophosphatemia with increased Ca and calcitriol
- 7
Exclude
- a
Other renal-tubular P-wasting syndromes:
Fanconi syndrome, myeloma, Wilson’s disease, cystinosis, more global tubular dysfunction, enzyme deficiencies, hypophosphatasemia, mineralization inhibitors, Al, Fl, bisphosphonates, Cadmium, Ca deficiency + hypophosphatemia
- b
Drug nephrotoxicity
- c
Ifosfamide (RX of solid tumors): hypophosphatemia + global tubular dysfunction
- a
- 8
Bone histomorphology:
Biopsy posttetracycline labeling shows osteomalacia
Increased unmineralized bone or osteoid surfaces
Increased mineralization time—distance between 2 tetracycline labels
- 9
Suspicious causative lesion
Detect: Serial physical examination or imaging
Document: Humoral factor production by venous assay
Remove: Resultant complete symptomatic and biochemical remission
OTHER DIAGNOSTIC CONSIDERATIONS
TIO may be biochemically indistinguishable from X-linked hypophosphatemic rickets (XLHR), autosomal dominant hypophosphatemic rickets (ADHR), and hereditary hypophosphatemic rickets. The latter is distinguished from XLHR and ADHR by hypercalciuria and elevated serum calcitriol (see Table 36-1 ) (see Box 36-3 ). However, XLHR and ADHR typically involve children and have occasional delayed age onset, while XLHR is associated with osteosclerosis, calcified entheses, infrequent fractures, and no muscle weakness. Other renal phosphate wasting syndromes, such as Fanconi syndrome, multiple myeloma, Wilson disease, and cystinosis, display more global renal tubular defects causing hypercalciuria, elevated serum calcitriol, amino-aciduria, glycosuria, and renal tubular acidosis. Chemotherapeutic agents, such as ifosfamide, may also be nephrotoxic with more global biochemical aberrations, again excluding TIO.
EXPERIMENTAL EVIDENCE FOR A HUMORAL SUBSTANCE
Biochemical aberrations produced by TIO lesions have been attributed to a humoral factor or factors initially referred to as phosphatonin. Animals injected with tumor extracts or transplanted cells from TIO lesions inhibit phosphorus transport in vitro and produce phosphaturia and hypophosphatemia in vivo.
Tissue culture media derived from them also inhibit phosphate uptake in opossum kidneys whose proximal tubules, as in humans, normally reabsorb phosphate. A humoral substance in cultured kidney cells, with other factors, also inhibits 1-alpha–hydroxylase enzyme with resultant serum 1,25-dihyroxy–vitamin D reduction.
While slow growth and loss of humoral activity in tissue cultures have heretofore hampered phosphatonin investigations, recent strategy has centered on analyzing gene expression profiles of TIO lesions. One of several genes involved in producing a phosphaturic substance has been identified as FGF23 of the fibroblast growth family. It inhibits phosphorus transport in cultured renal proximal tubular epithelium, reduces serum phosphorus, and increases its excretion in injected mice who become hypophosphatemic when chronically exposed. Conversely, FGF23-deficient mice exhibit hyperphosphatemia, hypercalcemia, elevated calcitriol, growth retardation, and premature death. While low FGF23 levels may be found in normal human tissues and serum and are occasionally slightly higher in polyostotic fibrous dysplasia, FGF23 is most highly expressed in TIO tumors. It may also operate in XLH due to a mutation in the PHEX gene, which encodes endopeptidase whose lost activity leads to phosphorus wasting. FGF23 has been cited as a substrate for PHEX with resultant failure of FGF23 cleavage said to enhance its activity. FGF 23 plays a role in several distinct disorders of renal P wasting.
- 1
In TIO tumors, strong expression of FGF23 inhibits phosphorus reabsorption by proximal renal tubules and down-regulates 25-hydroxy–vitamin D-1 alpha-hydroxylase with resultant hypophosphatemia and osteomalacia.
- 2
In ADHR, FGF23 gene mutation enhances its biologic activity by becoming resistant to proteolytic cleavage, again resulting in hypophosphatemic rickets.
- 3
In XLH, the PHEX mutation directly or indirectly increases circulatory FGF23, similarly interfering with proximal renal tubular phosphaturic activity. FGF23 is not the only factor in TIO tumors that may affect renal phosphate handling and bone mineralization. Another phosphatonin candidate due to mutations in NPT2, a major sodium-phosphate cotransporter in renal proximal tubules that reabsorbs up to 85% of glomerular filtered phosphorous, has been proposed as a new hypophosphatemic disorder. However, hypercalciuria and elevated calcitriol distinguish it from TIO.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree