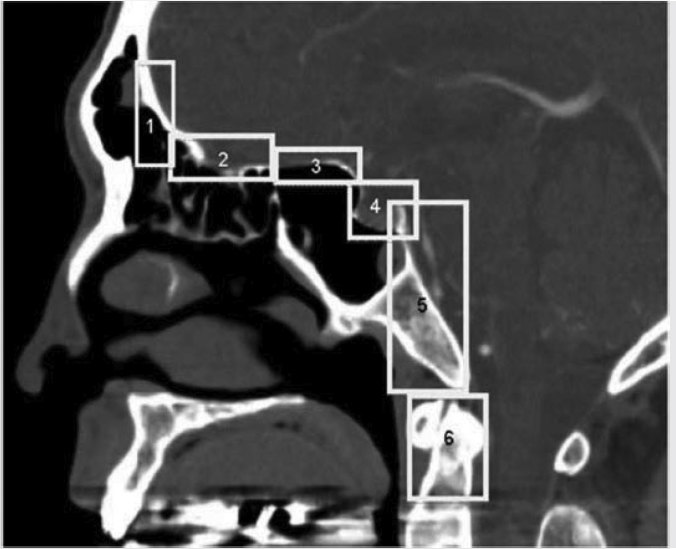
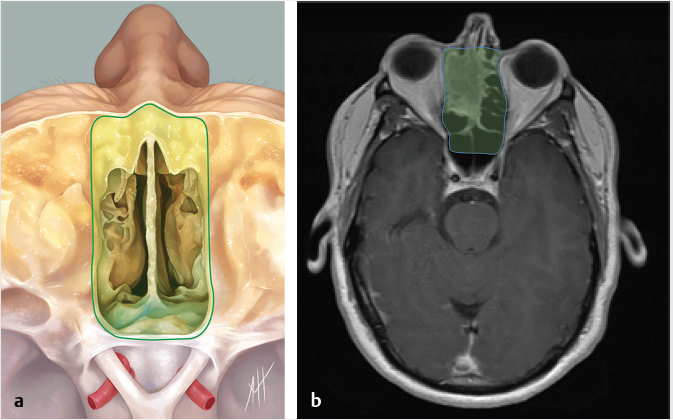
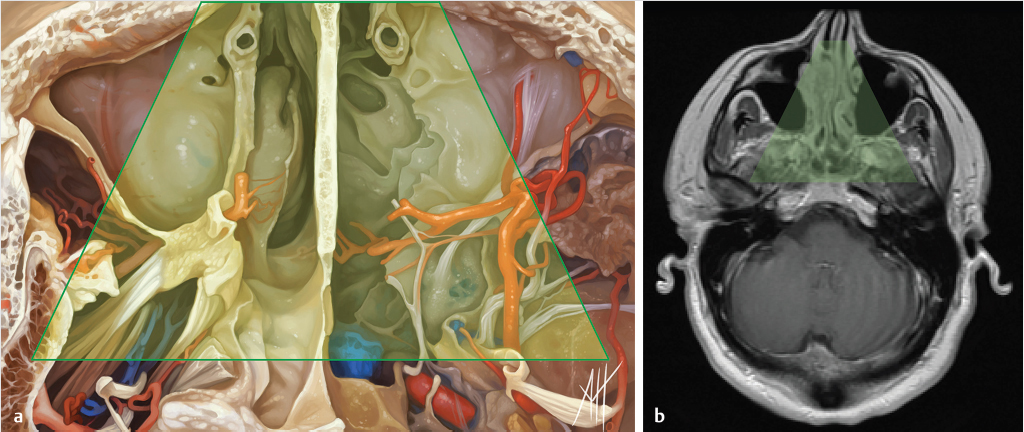
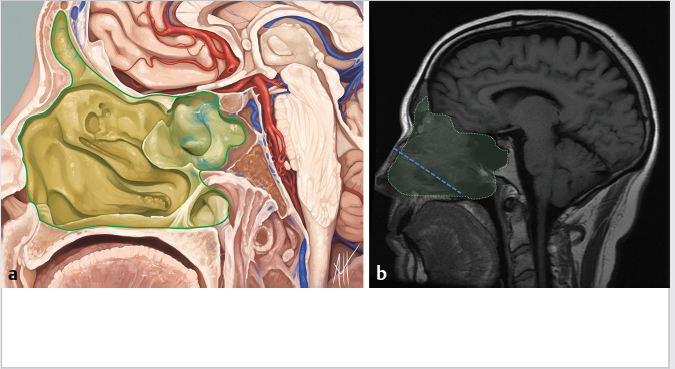
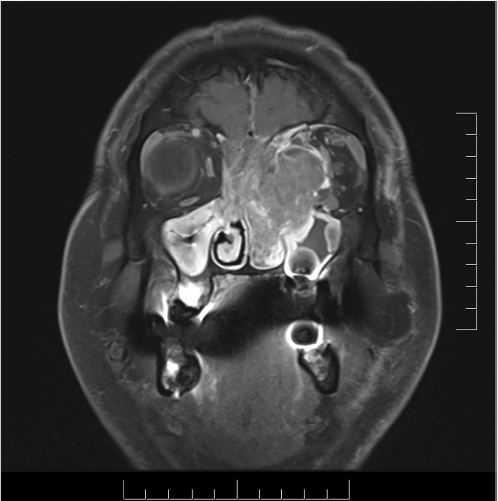
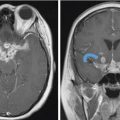
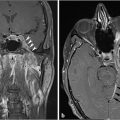
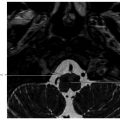
5 Open and Endoscopic Approaches to the Sinonasal Cavity and Skull Base Surgical access to the sinonasal cavity and skull base is intrinsically challenging given their proximity to critical anatomy and neurovascular structures. The ideal surgical approach to the sinonasal cavity and skull base should provide adequate exposure for complete surgical resection and subsequently for appropriate reconstruction. Cancers of the nasal cavity and paranasal sinuses make up a small subset of these head and neck tumors, but given their location and proximity to important structures, they may be some of the most difficult to manage. The underlying goal of treatment remains gross total tumor eradication, whether through surgery, radiation, chemotherapy, or a combination of modalities. As surgical, radiation and imaging techniques have improved, so have survival, functional outcomes, and quality of life. More recently, endoscopic endonasal approaches have been adopted as a strategy in surgical management of these tumors. Endoscopic endonasal approaches offer improved visualization and the avoidance of external facial incisions and external bony osteotomies for tumor access. As experience with endoscopic techniques and technology have progressed, an increasing amount of skull base tumors have become accessible. However, the ultimate goals of surgical management, which include the extirpation of tumors, with negative surgical margins while maintaining patient quality of life, have not changed. Isolated frontal sinus pathology is rare. Most commonly these include inflammatory sinus disease. Other processes include complications of inflammatory sinus disease such as mucocele, benign pathology such as osteomas, traumatic cerebrospinal fluid (CSF) leaks associated with frontal sinus fractures, and rarely isolated sinus malignancy. Pathology of the anterior cranial base includes sinonasal malignancies and metastatic disease. Benign histopathology includes osteomas, cavernous hemangiomas, and inverted papillomas, among others. Anterior cranial base meningiomas of the olfactory groove, planum sphenoidale, or tuberculum sellae also arise in this location. Iatrogenic, congenital, or idiopathic injuries or deficiencies in the skull base resulting in CSF leak and/or meningoencephaloceles may arise here. Sellar pathology most commonly arises in the pituitary gland in the form of pituitary adenomas. Other pathology in these locations includes craniopharyngiomas, Rathke’s cysts, and meningiomas. Nasopharyngeal carcinoma is the most common pathology of the nasopharynx, although other rare pathologies such as Tornwaldt cysts and minor salivary gland tumors may arise here. Clival tumors chordomas, chondrosarcomas, as well as other infectious processes such as skull base osteomyelitis can develop in this location. Pathology of the upper cervical spine may be accessed by endoscopic techniques in rare instances, but commonly requires open procedures. Pathologies of this location include chordomas and inflammatory processes such as rheumatoid panus associated with basilar invagination. Petrous apex pathologies include inflammatory disease such as cholesterol granulomas and malignant processes such as chondrosarcoma and metastatic disease. Endoscopic anatomy, from the surgical perspective, is conceptualized differently than traditional open surgical approaches and has been termed “inside out anatomy.” For surgeons facile with open transfacial or transcranial approaches, this involves an understanding of nasal and paranasal sinus anatomy as the focal point of surgical access. With endoscopic approaches, skull base pathology is approached through corridors usually created by the surgeon. Endoscopic surgical anatomy maybe conceptualized as approaches of the “sagittal plane” and those of the “coronal plane.” “Because of the structures in the mediolateral plane, coronal plane approaches may be associated with more potential collateral damage associated with the surgical approach. As such, it has been suggested that surgeons undertaking these procedures adopt a graduated approach beginning with cases involving the sagittal plane early in their experience before undertaking cases involving the coronal plane.1,2 The sagittal plane is divided into several surgical modules including transfrontal, transcribriform, transplanum, transsellar, transclival, and transodontoid approaches ( Fig. 5.1 Endoscopic endonasal approaches in the sagittal plane. Approaches can be divided into (1) transfrontal, (2) transcribriform, (3) transplanum, (4) transsellar, (5) transclival, and (6) transodontoid approaches. (Used with permission from Snyderman CH, Pant H, Carrau RL, et al. What are the limits of endoscopic sinus surgery? The expanded endonasal approach to the skull base. Keio J Med. 2009;58(3):152–160.) Anatomically, endoscopic access to the frontal sinus may be limited anteriorly and pathologies at the anterior and superior aspects of the frontal sinus often require open approaches. The frontal sinus outflow tract or frontal recess is bordered medially by the middle turbinate, anteriorly by the frontal process of the maxilla and frontal bone (frontal beak), laterally by the lamina papyracea, and posteriorly by the bulla ethmoidalis. The agger nasi air cell is the most anterior ethmoidal air cell and lies anterolateral to the frontal recess. Enlargement of the frontal recess often requires skeletonizing this air cell. The roof of the anterior skull base is formed by the cribriform plate medially and the fovea ethmoidalis laterally. The cribriform plate is often located inferior to the plane of the fovea ethmoidalis. These two structures are connected by an oblique bone known as the lateral lamella of the cribriform plate. Because of its relatively thin structure, the lateral lamella is often an area susceptible to iatrogenic injury and resultant CSF leaks. The risk for injury to this structure is thought to be related to its vertical height. The classification system of Keros describes the height of the lateral lamella, or the difference in height of the fovea ethmoidalis and the cribriform plate, as either type 1 (depth of 1–3 mm), type 2 (depth of 4–7 mm), or type 3 (8–16 mm).3 The greater the depth, the higher the risk of iatrogenic injury. Also, an asymmetrical skull base (between the left and right sides) poses a higher risk for iatrogenic injury. More posteriorly located is the sphenoid sinus which drains anteriorly into the nasal cavity through the sphenoethmoidal recess. The sphenoid ostium is located medial to the superior turbinate. The sphenoid sinus is the central point for many endoscopic skull base approaches. Also intimate with the sphenoid sinus are several critical structures such as the sella turcica, the internal carotid artery, and the optic nerve. The sphenoid sinus pneumatization may vary from individual to individual. The pneumatization of the sphenoid may be classified as a sellar, presellar, or conchal pneumatization pattern.4 The most pneumatized of these patterns is the sellar pattern, followed by presellar and conchal. Conchal pneumatization may pose challenges for surgical access in that anatomic landmarks are not readily visualized and the sella, carotid arteries, and optic nerves are often hidden in bone. When planning surgical approaches that require opening of the sphenoid sinus, surgeons must be aware of the presence of Onodi air cells. Onodi cells are posterior ethmoidal air cells that pneumatize superiorly and laterally above the sphenoid sinus. These cells, when present, can place certain anatomic structures, particularly the optic nerve, at risk. There are several limits to endoscopic approaches ( Fig. 5.3 Axial view of endoscopic limits of resection at the level of the maxillary sinus in (a) graphic image and (b) axial imaging (magnetic resonance imaging). Access to the lateral wall of the maxillary sinus as depicted in the above figure is limited but may be improved with a Caldwell-Luc approach or a Denker’s approach. The endoscopic approach provides good access to the pterygopalatine fossa and infratemporal fossa and can be further enhanced with the previous two modifications. Fig. 5.4 Sagittal view of endoscopic limits of resection in (a) graphic image and (b) sagittal imaging (magnetic resonance imaging). The anterior extent of surgical access is the posterior table of the frontal sinus. Tumor extension into the anterior table of the frontal sinus is often difficult to access endoscopically. Anterior extension of tumors into the soft tissues of the nose and nasal bones often requires open approaches for access. The posterior extent depicted here are for malignant sinonasal tumors. Further posterior access to the planum, tuberculum, and clivus is possible. Similarly, superior extension depicted here is for malignant sinonasal tumors. Further superior dissection is possible. The dotted line depicts the nasopalatine line. Understanding of the anatomy for open surgical approaches requires understanding of the function of the anatomic structures in the surgical field, as well as the course of critical neurovascular structures, and the potential spaces in which these structures may reach a confluence. The nasal cavity warms and humidifies air, while functioning to also filter out larger particles. The nasal septum, made up of cartilage and bone, comprises the midline structure of the nose. Attached on the lateral walls are the paired inferior, middle, and superior turbinates. Occasionally, a supreme turbinate is also present. Olfactory mucosa helps in the detection of odors, and is located along the skull base and superior septum. The nasal cavity meets the nasopharynx at the choana posteriorly, where the adenoid tissue is located. Classically, there are four paired air-filled sinuses: the maxillary, ethmoid, frontal, and sphenoid sinuses. The maxillary sinus (“antrum of Highmore”) drains into the middle meatus, specifically the infundibulum. The entrance to the infundibulum is the crescent-shaped hiatus semilunaris, formed by the uncinate process and the bulla ethmoidalis. Ohngren’s line represents an imaginary line that divides the maxillary sinus into anterior and posterior compartments. It is formed by drawing a line from the medial canthus to the angle of the mandible. Tumors inferior to this line are prognostically more favorable and generally referred to as infrastructural tumors, whereas tumors above this line are generally referred to as suprastructural tumors. Beyond the posterior wall of the maxillary sinus is a fat-filled space known as the pterygopalatine fossa. This fossa contains several important structures including the internal maxillary artery, the pterygopalatine ganglion, the Vidian nerve, the infraorbital nerve and V2 nerve, the palatovaginal nerve, and the descending palatine nerve. There are seven foramina that communicate with this space, all of which provide potential routes of tumor spread. These foramina include foramen rotundum (V2), Vidian canal, the palatovaginal canal, inferior orbital fissure, sphenopalatine foramen, the pterygomaxillary fissure, and the greater palatine canal. Lateral to the pterygopalatine fossa is the infratemporal fossa. This is separated from the pterygopalatine fossa by the pterygomaxillary fissure. The borders of this space include the maxillary tuberosity anteriorly, the temporal bone posteriorly, the greater wing of the sphenoid bone superiorly, the medial pterygoid muscle inferiorly, the mandibular ramus laterally, and lateral pterygoid plate medially. Diagnostic imaging of the skull base is critical to adequately delineate tumor extent, as well as relationships between tumors and critical anatomy. Cross-sectional imaging can be obtained with either computed tomography (CT) and/or magnetic resonance imaging (MRI). CT provides detailed imaging of bony structures as well as information about bony erosion, remodeling, and/or invasion of bony structures surrounding the paranasal sinuses and nasal cavities. Structures such as the walls of the maxillary sinus, orbits, pterygoid plates, hard palate, or the clivus and skull base should be examined for bony erosion or invasion. CT may further aid in identifying dehiscence of the internal carotid artery or optic nerves and bony erosion or invasion of the skull base and all the cranial nerve foramina. Contrast-enhanced CT scans may add further detail to noncontrast studies and are useful in staging the neck for regional spread of tumors. MRI adds superior soft-tissue delineation in comparison to CT. T1-weighted imaging may help identify bone invasion particularly in marrow-rich bone such as the clivus. MRI will also show muscle denervation secondary to cranial neuropathies. Fatty infiltration of the pterygoid or temporalis muscles, for example, may suggest a loss of function of the motor branch of the trigeminal nerve due to chronic denervation. T2-weighted imaging can also help differentiate between trapped mucous secretions and soft-tissue tumor. Secretions are typically hyperintense on T2-weighted imaging in comparison to solid tumor, which is mildly hyperintense to intermediate in signal. Contrast-enhanced MRI with gadolinium will also help delineate sinonasal tumors with enhancement of the lesion. Contrast may further aid in detecting perineural spread of nerves intracranially. The addition of special sequences such as the fast imaging employing steady-state acquisition (FIESTA) may aid in detecting cranial nerve tumor spread. Other sequences such as the fluid attenuated inversion recovery (FLAIR) sequence are used to identify brain edema secondary to parenchymal invasion. Orbital invasion can also be seen on MRI ( The short tau inversion recovery (STIR) sequence may be used to detect orbital fat invasion. Tumor adjacent to the periorbita, extraocular muscle involvement, and orbital fat obliteration may suggest orbital invasion.6 Periorbita is typically hypointense on T2 and contrast-enhanced T1-weighted images relative to tumor. Loss of this plane on MRI may further suggest orbital invasion. Fig. 5.5 T1-weighted magnetic resonance imaging with gadolinium showing a sinonasal tumor extending into the left orbit. Positron emission tomography (PET)/CT scanning with 18F-fluorodeoxyglucose (FDG) can be used both in staging and restaging tumors. PET scans are commonly used in the detection of recurrences in the surveillance setting, particularly in health care settings where it is readily available. Special nuclear imaging such as technetium-99 m, gallium-67, indium-111 scans can also be helpful in certain situations to better help delineate infectious processes such as osteomyelitis of the skull base. Technetium-99 bone scans are typically positive focally shortly after an acute infection. These scans, however, may remain positive long after the clearance of the infectious process. Gallium-67 scans, on the other hand, may be helpful in monitoring responses to therapy and may be used to monitor the progress of infectious processes. Indium-111-labeled scans are helpful in identifying acute or chronic processes but may be more specific than the other two modalities in identifying infection. Advantages of the endoscopic approach include superior tumor visualization with high definition magnification of the surgical field. In addition, entirely endonasal approaches avoid external facial incisions. Compared to the open approach, endoscopic approaches may be associated with fewer complications and shorter hospital stays.7,8,9 The ideal candidates for an endoscopic resection are those patients with a tumor confined to the nasal cavity or ethmoid sinuses with limited anterior, lateral, and inferior extension. Tumor erosion through the posterior wall of the frontal sinus may be resectable, but extensive tumor extension into the frontal sinus abutting the anterior wall requires an open approach through either a frontal craniotomy or an osteoplastic flap. To access lateral tumors, a Denker’s maxillotomy or a Caldwell-Luc approach can be combined with endoscopic approaches to reach the lateral wall of the maxillary sinus, pterygopalatine fossa, and infratemporal fossa. Endoscopic anterior craniofacial resection is typically used for midline tumors of the nasal cavity or ethmoid sinuses with skull base involvement.10,11,12,13,14 Preliminary experience with this approach was developed around the management of esthesioneuroblastomas (olfactory neuroblastoma) because of their favorable location and biology. However, many sinonasal malignancies as well as anterior skull base meningiomas may now be accessed with this approach. The endoscopic anterior craniofacial resection combines an endoscopic approach (
5.1 Introduction
5.2 Skull Base Pathology
5.2.1 Frontal Sinus
5.2.2 Anterior Cranial Base
5.2.3 Sella/Suprasellar
5.2.4 Nasopharynx/Clivus
5.2.5 Upper Cervical Spine
5.2.6 Petrous Apex
5.3 Endoscopic Surgical Anatomy
 Fig. 5.1). The coronal plane may be subdivided into anterior, middle, and posterior coronal planes.2 The anterior coronal plane is primarily composed of the transorbital approach. The middle coronal plane is composed of transpterygoid, infrapetrous, and suprapetrous approaches, as well as approaches to the cavernous sinus. The posterior coronal plane includes approaches to the parapharyngeal space and jugular foramen.
Fig. 5.1). The coronal plane may be subdivided into anterior, middle, and posterior coronal planes.2 The anterior coronal plane is primarily composed of the transorbital approach. The middle coronal plane is composed of transpterygoid, infrapetrous, and suprapetrous approaches, as well as approaches to the cavernous sinus. The posterior coronal plane includes approaches to the parapharyngeal space and jugular foramen.
 Fig. 5.2,
Fig. 5.2,  Fig. 5.3,
Fig. 5.3,  Fig. 5.4). Anteriorly, the anterior table of the frontal sinus may be difficult to access. Although the posterior table is often accessible, this may provide challenges in terms of tumor resection and reconstruction. Superolaterally, the midline of the orbit is generally considered the farthest lateral access that can be achieved with an endoscopic approach. Inferolaterally, access to the pterygopalatine fossa, infratemporal fossa, and middle cranial fossa can be achieved through an endonasal approach. Further lateral access can be achieved with a Denker’s modification or with a Caldwell-Luc modification (these modifications are described in section “Endoscopic Lateral Approaches”). Inferiorly, the palate serves as the inferior border of dissection. Lesions involving the palate often require open approaches for complete resection. The inferior limit of dissection posteriorly is limited by fixed bony structures with the nasal bones superiorly and the hard palate posteroinferiorly. The so-called nasopalatine line is an imaginary line connecting these two anatomic structures and serves as a theoretical boundary for inferior dissection (
Fig. 5.4). Anteriorly, the anterior table of the frontal sinus may be difficult to access. Although the posterior table is often accessible, this may provide challenges in terms of tumor resection and reconstruction. Superolaterally, the midline of the orbit is generally considered the farthest lateral access that can be achieved with an endoscopic approach. Inferolaterally, access to the pterygopalatine fossa, infratemporal fossa, and middle cranial fossa can be achieved through an endonasal approach. Further lateral access can be achieved with a Denker’s modification or with a Caldwell-Luc modification (these modifications are described in section “Endoscopic Lateral Approaches”). Inferiorly, the palate serves as the inferior border of dissection. Lesions involving the palate often require open approaches for complete resection. The inferior limit of dissection posteriorly is limited by fixed bony structures with the nasal bones superiorly and the hard palate posteroinferiorly. The so-called nasopalatine line is an imaginary line connecting these two anatomic structures and serves as a theoretical boundary for inferior dissection ( Fig. 5.4).5
Fig. 5.4).5
5.4 Open Surgical Anatomy
5.5 Skull Base and Sinonasal Imaging
 Fig. 5.5).
Fig. 5.5).
5.6 Surgical Approaches
5.6.1 Endoscopic Surgical Approaches
Endoscopic Anterior Craniofacial Resection
 Fig. 5.6) along with the access of the traditional transfacial/transcranial open craniofacial resection. This allows for resection of skull base pathology as well as intracranial resection when necessary. Limits of resection for this approach include the frontal sinus anteriorly, the planum sphenoidale posteriorly, and the midline of the orbits laterally. Clinical and/or imaging findings that preclude the use of an endoscopic resection (thus requiring an open approach) include the following:
Fig. 5.6) along with the access of the traditional transfacial/transcranial open craniofacial resection. This allows for resection of skull base pathology as well as intracranial resection when necessary. Limits of resection for this approach include the frontal sinus anteriorly, the planum sphenoidale posteriorly, and the midline of the orbits laterally. Clinical and/or imaging findings that preclude the use of an endoscopic resection (thus requiring an open approach) include the following:
Radiology Key
Fastest Radiology Insight Engine