© Springer International Publishing AG 2017
Pietro Ruggieri, Andrea Angelini, Daniel Vanel and Piero Picci (eds.)Tumors of the Sacrum10.1007/978-3-319-51202-0_1010. Osteoid Osteoma of the Sacrum
(1)
Department of Orthopaedics and Orthopedic Oncology, University of Padova, Via N. Giustiniani, 3, Padova, 35128, Italy
10.1 Introduction
Osteoid osteoma is a benign bone-forming neoplasm, first described by Jaffe in 1935 [1]. Approximately 10% of all benign bone tumors are osteoid osteomas [2, 3]. It is found most frequently in the second to third decade of life, and there is a pronounced male predominance (2:1 male-to-female predilection). Any portion of the skeleton may be involved, but it is often found (50–60% of cases) in the long bones of the lower extremity [3] and (between 19 and 31% of the cases) upper extremity [4, 5], whereas about 20% occur in the spine [6]. Only 2% of spinal osteoid osteomas are found in the sacrum [2, 7].
10.2 Epidemiology, Presentation, and Diagnosis
Patients with osteoid osteoma typically present with a significant well-localized pain, which is often worse at night and is relieved by nonsteroidal drugs. This lesion is rarely painless, although absence of pain has been reported [8–10]. In some cases pain is not well localized by the patient and can be referred to a nearby joint or in a limb. There are two hypotheses about the source of the pain in osteoid osteoma: (1) abnormally high concentrations of prostaglandins (mainly prostaglandins E2 and I2) have been reported within the nidus, which causes local inflammation and vasodilation [11, 12], as well as strong immunohistochemical staining for cyclooxygenase-2 (COX-2) [13]; (2) pain may be mediated by the nerve fibers located in the reactive bone around the lesion [14–16]. Back pain is a common symptom and this fact may considerably delay the diagnosis of osteoid osteoma of the sacrum considering a lot of differential diagnosis. Complaints of pain are frequent and mean duration of symptoms is about 12 months [17]. In recent years, however, MRI or CT examination often is used to diagnose patients with back pain, shortening therefore the delay in diagnosis.
10.3 Imaging
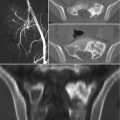
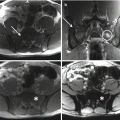
Tumors usually present as active, benign stage 2 lesions (Musculoskeletal Tumor Society) and most typically arise from the articular process of S1. Radiographically the majority of the osteoid osteomas were osteosclerotic with or without recognition of a nidus that appear as radiolucent area surrounded by a rim of dense reactive bone. The nidus of an osteoid osteoma is always less than 2 cm in diameter. As “giant osteoid osteomas” were historically defined, the rare cases in which the osteoid osteoma is large (up to 2 cm) retaining the same clinical, imaging, and histological features [2]. Sometimes osteoid osteoma may be obscured by reactive bone and may not be readily visible in a radiograph. In patients with persistent back pain, a bone scan should be obtained to check the existence of this lesion [2, 18]. Bone scintigraphy is constantly positive showing intense focal isotope uptake centered in a less intensely positive and diffused halo (double density sign) [2, 18]. The extent of the isotope uptake is significantly larger than the radiographic dimension of the nidus, encompassing the entire reaction about it. Thin-cut CT scans (1 mm sections) are excellent for diagnosis and precise localization of the nidus. Contrast is usually not needed. Central calcification may be observed within the osteolytic nidus. Sometimes there is a zone of radiolucency between the central area of mineralization and the surrounding reactive bone when ossification of the nidus begins. Some authors reported the use of dynamic contrast-enhanced CT to help differentiate osteoid osteoma from Brodie’s abscess, demonstrating vascular flow within the lesion [19]. MR imaging usually shows high signal intensity area in the muscles or bone around the lesion, or both were seen on the T2-weighted image and may be useful for localizing rare intramedullary or periarticular surface osteoid osteomas [20]. However MRI is inferior to CT in depicting the nidus and strongly influenced by the inflammatory reaction of the surrounding tissue.
10.4 Pathology
Grossly, the central nidus of osteoid osteomas appears small and hyperemic, pink to cherry red colored. Histologically, the central portion is composed of abundant, loose, fibrovascular connective tissue between contorted amorphous osteoid and woven trabeculae. Numerous osteoblastic rimming, osteoclasts surrounding the bony trabeculae, and dilated capillaries are observed. The surrounded host bone appears as reactive woven and trabecular bone, more or less mature and sclerotic. Barlow et al., analyzing histologically 10 osteoid osteomas and 20 osteoblastomas (10 spinal and 10 non-spinal), concluded that both tumors are innervated bone-forming lesions which share histomorphological and immunohistochemical features, supporting the view that separate classification is unjustified [21].
10.5 Treatment
In some cases, osteoid osteoma may be self-limiting: rare cases managed by nonsteroidal anti-inflammatory drugs reported pain relief after some years and nidus regression; however, its course is unpredictable and may be protracted. Once the osteoma osteoid has been diagnosed, the physician should discuss nonoperative and operative treatments with the patient. Most patients initially are treated with long-term oral administration of nonsteroidal anti-inflammatory drugs and analgesic drugs [2, 22–25]. If this treatment is ineffective, there are many other alternatives available [26–34].
10.5.1 Radiofrequency Thermal Ablation (RFTA)
Technological advances have allowed for the development of various percutaneous procedures to treat osteoid osteoma. Percutaneous radiofrequency thermal ablation (RFA) has proven to be an effective, reliable, minimally invasive technique in the treatment of osteoid osteoma [32, 35]. The curative approach is operative removal or deactivation of the nidus with RFTA [36], and most of the patients experienced complete disappearance of the pain. If the treatment is not complete, pain persists and additional operative intervention is necessary [37]. RFTA in the spine appears as a safe technique if the lesion is not adjacent to neural structures and the bone cortex is intact [32, 38]. Close proximity of the RFA probe to a neurovascular bundle is a relative contraindication, but morbidities of other treatment options should be considered.
10.5.2 Surgery
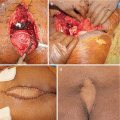
Conventional surgical treatment consists of en bloc resection or curettage of the lesion, which is challenging in certain anatomical locations, such as the acetabulum, femoral neck, spine, and sacrum. Although preoperative radiologic identification of the nidus in osteoid osteoma is not difficult with bone scan, CT, or MRI, localization of the nidus during surgery is difficult [7, 39]. Kirchner et al. [39] reported a probe-guided technique for intraoperative detection of osteoid osteoma and curettage: they injected 99 mTc-methylene diphosphonate 2–3 h before surgery and then identified the point of the maximum count rate during surgery and started curettage of the lesion. They concluded that this method seems to be useful to obtain confirmation of complete removal of the lesion by a continuous check of the count at the same point. If open techniques are preferred, extended intralesional curettage should be performed, and particular care should be taken to remove the entire nidus. Usually no reconstruction may be needed.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree