Fig. 21.1
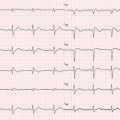
(a, b) Echocardiographic study in a 42-year-old woman with peripartum cardiomyopathy associated with preeclampsia. Parasternal long-axis view (a), diastolic frame, of the first echocardiogram postdelivery documenting severe left ventricular (LV) hypertrophy (septal wall thickness 17 mm, posterior wall thickness 14 mm), with diffuse hypokinesis and severe systolic dysfunction [fractional shortening 15 %, ejection fraction (EF) 32 %]. After 6 months of therapy with beta-blocker and angiotensin-converting-enzyme inhibitor, echocardiography in the parasternal long-axis view, diastolic frame (b) shows normal LV wall thickness (septal wall thickness 9 mm, posterior wall thickness 9 mm), with normal regional wall motion and systolic function (fractional shortening 39 %, EF 64 %)
There are few data about the use of advanced echocardiographic technologies in this disease. A similar counterclockwise rotation during systole of both the base and the apex at speckle-tracking analysis was reported in one case of peripartum CMP associated with LV NC [7]. This subtle deformation abnormality has been interpreted as secondary to dysfunction of basal and midwall subepicardial fibers, with resultant predominant effect exerted by the subendocardial fibers in this region. These abnormalities were reversible after optimal medical therapy. However, further investigation is required.
Studies on cardiac magnetic resonance (CMR) in the acute phases of peripartum CMP show the presence of edema at T2 sequences and on early gadolinium enhancement, suggesting an inflammatory etiology for this condition. In addition, late gadolinium enhancement (LGE) seems to identify cases with unfavorable prognosis [8, 9].
Although peripartum CMP shares many features with other forms of nonischemic CMP, women affected by this disease have a higher rate of spontaneous recovery of LV function [5]. Monitoring patients after diagnosis should include serial echocardiograms to identify improvement in systolic function in response to conventional HF medical treatment. Less severely impaired LV EF and lower LV dimensions at the time of diagnosis are predictors of complete LV function recovery and good outcome [10].
Previous studies assessed prognostic implications of LV contractile reserve at initial diagnosis and demonstrated that improved LV EF during dobutamine stress echocardiography accurately correlates with subsequent recovery of LV function and confers a benign prognosis to this CMP [11].
The existing literature indicates that many patients with peripartum CMP actually have myocarditis. The diagnosis of myocarditis is more suspicious in the presence of progressive worsening of LV dilation and systolic dysfunction at serial echocardiographic evaluations. In these cases, endomyocardial biopsy (EMB) can be useful to modify therapeutic strategies.
Finally, one important question asked by women with a history of this disease is whether they can safely get pregnant again. For women with persistently reduced LV EF, there is a substantial risk of recurrent HF. On the other hand, for women who recover, the risk is much lower and can be further stratified by a stress echocardiogram: in the presence of normal contractile reserve, the risk of recurrent disease or HF seems to be low [4].
21.3 Tako-Tsubo Cardiomyopathy
Tako-tsubo, or stress-induced CMP, is an acute, usually reversible, disorder of the heart characterized by transient LV dysfunction [12–15]. Clinical presentation is usually similar to an acute coronary syndrome, with precordial pain and dyspnea. Other symptoms may include palpitations, syncope, or even cardiac arrest or sudden death (SD). A trigger, such as a stressful physical or psychological event, can usually be detected in 27–100 % of cases. Many diagnostic criteria have been formulated, but the most widely accepted are those of the Mayo Clinic in the United States [13], which require normality of the epicardial coronary arteries. Tako-tsubo syndrome may be confused with stress syndrome caused by underlying pheochromocytoma, and attention should be paid to this clinically important differential diagnosis. The etiology and pathogenesis are presently unknown. One possibility is stress-induced catecholamine release, producing cardiac stunning through direct toxic damage to myocytes and/or vasoconstriction with ischemia [16]. The apical region is the most vulnerable area, probably due to the large number of adrenergic receptors [14]. Treatment is symptomatic and is determined by the complications occurring during the acute phase. Complications are estimated to occur in 19 % of cases, mortality varies between 0 and 12 % [15], and recurrence is rare.
Echocardiography plays a major role in achieving this diagnosis. In fact, the most salient feature of this disease is the unusual LV contractile pattern noted at the time of admission. Typically, it is characterized by a transient hypokinesis, akinesis, or dyskinesis of the LV apical and mid segments, usually with compensatory hyperkinesia of the basal portions (Fig. 21.2, Clip 21.3a) [17]. Moreover, regional wall motion abnormalities (WMA) extend beyond a single epicardial vascular distribution [18]. Global contractile LV function is usually significantly impaired. The time course for cardiac function improvement is variable, from a few days to several weeks; however, LV function typically recovers completely (Fig. 21.2, Clip 21.3b).
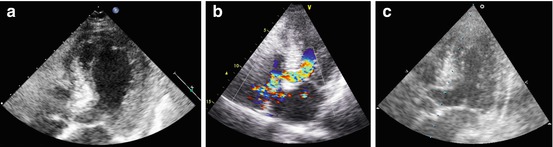

Fig. 21.2
(a–c) A 79-year-old woman with tako-tsubo-like cardiomyopathy. Echocardiography at the time of admission shows left ventricular (LV) apical akinesis, hypokinesis of the mid segments with compensatory hyperkinesis of the basal portions, and moderate systolic dysfunction (a) in the apical four chamber view, systolic frame. Apical long-axis view (b) recorded with color-Doppler imaging demonstrates marked turbulence in the LV outflow tract (OT), consistent with dynamic LV OT obstruction in the presence of a sigmoid interventricular septum. An echocardiogram performed 4 months later shows complete recovery of LV wall motion and systolic function (c) in the apical long-axis view, systolic frame
Atypical forms of stress-induced CMP have been increasingly reported. Transient midventricular ballooning with preserved basal and apical contractility (inverted tako-tsubo CMP) has been described [19]. Additionally, LV apical thrombosis may occur during the early stage of the disease due to transient apical akinesis and aneurysm formation [20]. The use of contrast agents can be particularly helpful to highlight apical thrombosis and if there is a difficult acoustic window.
Dynamic LV outflow tract (OT) obstruction is a relatively common complication of stress-induced CMP, with a reported incidence of >10 % (Fig. 21.2, Clip 21.3c) [21]. There are probably some predisposing factors to LV OT obstruction, i.e., sigmoid interventricular septum or a small LV or LV OT; more importantly, this complication is frequently precipitated by certain conditions, such as hypovolemia, ionotropic drugs, and counterpulsation.
Stress-induced CMP is also frequently complicated by acute mitral regurgitation (MR). Systolic anterior motion (SAM) of the mitral valve (MV) is typically associated with LV OT gradient and accounts for one half of acute MR. MV tethering with increased valve tenting area the in presence of LV systolic dysfunction and LV enlargement has been proposed as an alternative mechanism responsible for MR in this disease [22].
Right ventricular (RV) involvement and dysfunction is relatively common and is associated with lower LV EF [23]. Conversely, ventricular rupture is an extremely rare life-threatening complication of stress-induced CMP. There are a few cases reported in literature, principally in elderly female patients [24].
Myocardial viability and contractile reserve could be evaluated with low-dose dobutamine stress echocardiography in suspected stress-induced CMP. However, particular caution must be taken considering that increasing doses of dobutamine can worsen LV OT gradient. Furthermore, the possible occurrence of tako-tsubo CMP as a complication of dobutamine stress echocardiography has been reported [25].
Finally, if transthoracic echocardiography (TTE) is limited by poor acoustic windows, transesophageal echocardiography (TEE) can provide clearer image quality and allows careful evaluation of MV anatomy and full definition of the MR [17].
Advanced echocardiographic techniques could be useful in diagnosis and prognostic stratification of tako-tsubo CMP. Both 2D and 3D imaging allows recognition of the contractile pattern, mainly when the apex is involved; 3D imaging may allow better visualization of contracting and noncontracting segments when medial segments are involved. Myocardial contrast echocardiography seems to be a useful tool by which to clarify the mechanisms involved the myocardial injury and to distinguish stress-induced CMP from acute coronary syndrome. In one study, myocardial perfusion was relatively preserved in patients with tako-tsubo CMP compared with those with myocardial infarction. The authors of that study concluded that myocardial contrast echocardiography had a high sensitivity, specificity, and positive and negative predictive values for detecting stress-induced CMP (91, 86.2, 83, and 93 %, respectively) [26]. In addition, transient impairment of coronary flow reserve, assessed by Doppler coronary flow analysis during dipyridamole test, has also been reported in this disease [27]. Finally, at 2D strain by speckle-tracking analysis, a progressive decrease of longitudinal strain values from base to apex was reported in patients with classical stress-induced CMP [17].
CMR in this CMP exhibits the characteristic finding of transmural high T2 signal in the midanterior wall and apical segments, matching the distribution of WMA [28, 29]. This abnormality can persist after 3 months from the acute event although is substantially decreased compared with the acute phase [30]. Myocardial inflammation may also be found at global relative enhancement and quantitative T1 mapping sequences [31, 32].
Patients with tako-tsubo CMP usually do not exhibit hypoenhancement at first-pass perfusion [33], or by LGE [34, 35], thus helping differentiate this condition from acute myocardial infarction with spontaneous coronary recanalization or acute myocarditis [35]. When LGE is found, it is focal or patchy and has decreased signal intensity compared with ischemic LGE [36, 37]. This finding is usually indicative of a more severe acute condition, which tends to resolve within 6 months [38].
CMR data from a large multicenter registry of patients with tako-tsubo CMP revealed four distinct patterns of regional ventricular ballooning [36]:
Apical (82 %)
Biventricular (34 %)
Midventricular (17 %)
Basal (1 %)
Single-photon-emission CT (SPECT) findings include reduced apical perfusion assessed with technetium-99 m (99mTc)-tetrofosmin in the acute phase, which recedes during follow-up [39–41]. Severely impaired myocardial fatty-acid metabolism assessed with 123I-beta-methyl-p-iodophenyl pentadecanoic acid (123I-BMIPP) [42–44], and apical sympathetic dysinnervation in the acute phase assessed by 123I-metaiodobenzylguanidine (MIBG) uptake [43, 45–47] were also found, mismatched from perfusion defects.
Positron emission tomography (PET) studies also report perfusion/metabolism mismatch involving the apical akinetic area assessed by [18F]-fluorodeoxyglucose (FDG) and [13N]-ammonia or by thallium-201 radiochemical thallium chloride (201Tl) SPECT [48–50]. Glucose metabolism assessed by [18F]-FDG PET and sympathetic function assessed by [123I]-MIBG SPECT seem to be strictly correlated in affected segments [46].
In tako-tsubo CMP, LV apical WMA is typically transient and resolves over a period of days to weeks. Therefore, follow-up echocardiographic reevaluation is essential to monitor its resolution and recovery of LV systolic function. The prognosis is generally favorable; reported in-hospital mortality rates range from 0 to 8 % [18]. As it is difficult to predict subsequent occurrence of complications immediately after the onset of the disease, serial echocardiographic evaluations should be performed during the in-hospital period. There is no established consensus on how to manage stress- induced CMP, but early detection of complications, such as the occurrence of LV OT gradient or apical thrombosis, can protect against a poor outcome and may lead to some changes in therapeutic strategies.
In this disease, RV involvement is relatively common, and RV dysfunction is associated with lower LV EF, longer hospitalization, more complications, such as severe HF, and need for intra-aortic balloon pump and cardiopulmonary resuscitation [23]. LV EF at admission is one of the independent predictors of mortality [51]. Moreover, absence of LV function recovery within 1 week was an independent factor associated with mortality [52]. Other prognostic indicators of worse outcome are intraventricular thrombus formation and LV OT gradient occurrence [17]. Recurrence of stress-induced CMP occurs in approximately 10 % of patients [53] and is unpredictable from imaging data at first manifestation.
21.4 Tachycardia-Induced Cardiomyopathy
Tachycardia-induced CMP (TIC) is a frequent cause of reversible HF secondary to persistent supraventricular or ventricular arrhythmias and in the absence of pre-existing structural heart disease [54]. Early diagnosis is crucial for prompt re-establishment of sinus rhythm and normal heart rate, with consequent improvement or even normalization of myocardial function. The pathogenesis is still controversial: Elevated heart rate causes structural alterations of myocytes, mitochondrial abnormalities, oxidative stress, and loss of contractile tissue due to necrosis or apoptosis. Moreover, tachycardia reduces myocardial perfusion, leading to myocardial stunning, interstitial fibrosis, and neurohumoral variations with elevations in natriuretic peptide, endothelin, catecholamines, and the renin-angiotensin-aldosterone system (RAAS).
Strict clinical and echocardiographic follow-up is very important in order to recognize relapses, which tend to be more severe and life threatening. Furthermore, due to the fact that LV remodeling can occur despite EF and heart rate normalization, long-term treatment with beta-blockers and angiotensin-converting enzyme (ACE) inhibitors is recommended.
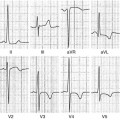
In TIC, imaging techniques are essential to exclude other causes of ventricular dysfunction and to assess its reversibility after normalization of atrial or ventricular rate; echocardiography, being widely available and inexpensive, is the cornerstone in establishing the presence of LV dysfunction and narrowing the list of differential diagnoses. LV systolic dysfunction is the typical model of presentation. However, other aspects can sustain the diagnostic hypothesis. Usually, myocardial thickness is preserved, indicating the absence of scar tissue. Moreover, some studies suggest that the absence of severe LV dilation may support the diagnosis of TIC rather than DCM accompanied by supraventricular tachycardia (Fig. 21.3, Clips 21.4a, 21.4b, 21.4c, and 21.4d) [55, 56]. In fact, Jeong et al. [55] reported that LV end-diastolic dimension ≤61 mm could predict TIC with a sensitivity of 100 % and a specificity of 71 % in patients with HF and tachyarrhythmia.
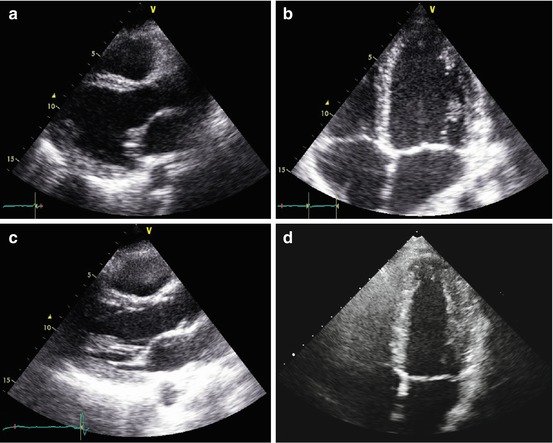

Fig. 21.3
(a–d) Echocardiographic study in a 32-year-old patient with atrial fibrillation (AF) and tachycardia-induced cardiomyopathy. Echocardiogram shows mild left ventricular (LV) dilation [end-diastolic diameter corrected for body surface area (BSA) 32 mm/mq), with normal wall thickness, diffuse hypo-akinesis, and severe systolic dysfunction [ejection fraction (EF) 22 %] in the parasternal long-axis (a) and apical four-chamber views (b), systolic frames. The echocardiogram performed 5 months after successful radiofrequency catheter ablation of AF shows normal LV dimensions (end-diastolic diameter corrected for BSA 26 mm/mq), wall motion, and systolic function (EF 60 %) in the parasternal long-axis (c) and apical four-chamber (d) views, systolic frames
LV diastolic dysfunction should be considered a component of TIC and is indeed sometimes the first expression of the disease [57]. However, it is particularly difficult to assess at Doppler in the presence of tachyarrhythmias. Contractile reserve at low-dose dobutamine stress echocardiography has been reported as a predictor of LV function recovery after catheter ablation of AF in TIC [58].
TEE can provide better image quality in patients with a poor acoustic window. Moreover, it is essential to identify left atrial appendage clots when restoring sinus rhythm is the chosen treatment. Finally, TEE and intracardiac echocardiography may be helpful during catheter ablation of arrhythmias, even without fluoroscopy [59]. There are a few data regarding the application of new and advanced echocardiographic technologies in the setting of TIC. Alterations in LV torsion have been reported in an experimental model [60]. Strain and strain-rate imaging can provide detailed regional and global LA functional assessment [61]. In patients who have undergone catheter ablation for atrial fibrillation, LA strain and strain rate during atrial filling (parameters of LA reservoir function) are known to be better predictors of sinus rhythm maintenance [62]. Real-time 3D TEE has been demonstrated to be feasible during ablation procedures, allowing fluoroscopy-free navigation and precise anatomical delineation of atrial structure [63]. CMR with LGE may differentiate TIC, which seldom shows LGE from primary CMP, in which LGE is frequent [64]. Myocardial glucose metabolism assessed by [18F]-FDG PET was found to be impaired in TIC [65].
The improvement of LV function after normalization of the heart rate is a hallmark of TIC. Therefore, multiple evaluations are necessary to identify changes in LV EF. Echocardiography is probably the most feasible and less expensive technique allowing close follow-up of these patients. The time course of improvement in biventricular systolic function has not been fully established; generally it can be observed soon after heart rate normalization [66]. If LV systolic dysfunction does not improve, other causes of impaired LV function must be reconsidered, justifying an “impure” form of TIC (possible association with dilated CMP, myocarditis, or ischemic heart disease).
It should be noted that patients with a history of successfully treated TIC are susceptible to a more severe CMP if the offending tachyarrhythmia recurs [67]. For this reason, patients should be periodically evaluated after complete LV function restoration.
21.5 Myocarditis
Myocarditis is an inflammatory disease of the myocardium and is diagnosed by established histological [68] (Fig. 21.4), immunological, and immunohistochemical criteria [69]. Most cases of myocarditis observed in clinical practice in the Western world are ascribable to viral infections and to the related reaction of the immune system. On the other hand, myocarditis can also be triggered by many specific causes, such as bacterial or parasitic infections, autoimmune diseases, hypersensitivity processes, hypercatecholaminergic states, drugs, toxic substances, and physical agents [69]. Clinical presentation of myocarditis is polymorphic, ranging from subclinical or benign forms to major clinical syndromes, such as severe HF or life-threatening ventricular arrhythmias. However, for simplification purposes, it can be categorized into three main patterns according to disease onset: HF, arrhythmias (hypokinetic or hyperkinetic, supraventricular or ventricular), and chest pain [70]. Moreover, myocarditis is characterized by great variability in its natural history evolution, ranging from resolution, to relapse, to development of DCM, or to unexpected SD. Patients presenting with chest pain and preserved LV function are projected through a favorable natural history, whereas those presenting with HF and significant LV dysfunction are generally characterized by a worse prognosis. On the other hand, it is important to emphasize that spontaneous (or therapeutically induced) improvement of ventricular function within a few months of symptom onset is described for 40–50 % of patients and identifies a subgroup with a fair long-term prognosis [70]. Advanced analysis of myocardial tissue samples collected with endomyocardial biopsy is thus far the only way to diagnose the disease definitively and to guide eventual treatment using immunomodulating drugs.
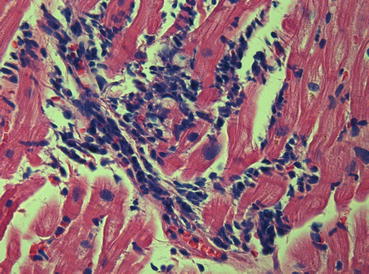

Fig. 21.4
Endomyocardial biopsy, histologic pattern, in a case of myocarditis (hematoxylin and eosin ×40). Severe lymphocytic infiltrates are present and are associated with “fraying” of myocytes (Dallas criteria)
In clinical practice, myocarditis and pericarditis may coexist. This clinical condition is known as perimyocarditis (or myopericarditis) [71]. A precise definition of perimyocarditis is still lacking. Consequently, the true incidence and prevalence of this disease remain unknown. The diagnosis is usually based on the combination of chest pain, pericardial rubs, increased inflammation indexes, electrocardiographic (ECG) and echocardiographic abnormalities (most frequently, pericardial effusion), with troponin release [71]. Clinical presentation is variable and may affect any or all cardiac chambers. Usually, chest pain is the main symptom and is associated with concomitant or recent flu-like syndrome, whereas ventricular/supraventricular arrhythmias and HF with LV dysfunction are rarely observed. Natural history is excellent (survival rate 100 %) during long-term follow-up, regardless of specific etiology and the presence of myocardial involvement (troponin release and WMA at echocardiography) at admission; relapses are relatively infrequent (<10 %) [72].
Typically, patients with myocarditis who present with recent onset of HF demonstrate at echocardiographic study a global impairment of ventricular function without significant remodeling of ventricular chambers, which often maintain a normal or augmented wall thickness and an ellipsoidal shape. Moreover, patchy WMA not corresponding to coronary distribution or ECG changes are often observed (Fig. 21.5, Clips 21.5a and 21.5b) [73, 74]. Additional elements, such as the presence of pericardial effusion, diastolic dysfunction, transient pseudohypertrophy of the ventricular wall, intraventricular thrombi [sometimes multiple and mobile (Fig. 21.6, Clips 21.6a, 21.6b, and 21.6c)] and alteration of the echocardiographic myocardial texture, may further strengthen, if correctly contextualized, the suspicion of myocarditis [73, 75–77]. On the other hand, patients who present with arrhythmias or chest pain and those with perimyocarditis usually exhibit ventricular chambers characterized by normal dimensions, regional wall motion, and global function, even if WMA or transient mild ventricular dysfunction may be observed [72, 78]. In such cases, careful re-evaluation of those abnormalities with a short-term follow-up is recommended in order to track disease evolution and promptly recognize potentially extensive myocardial involvement.
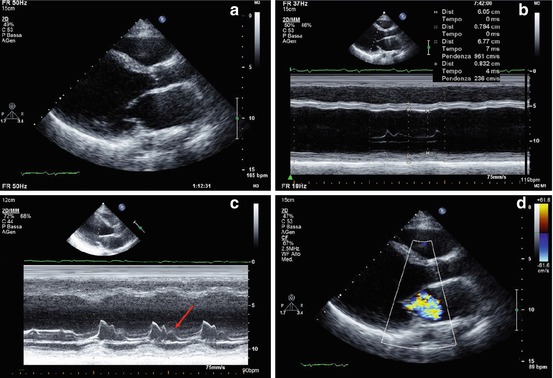
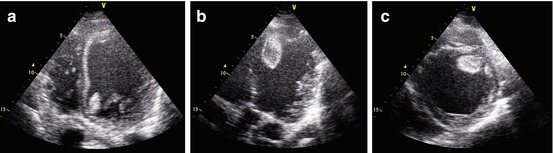

Fig. 21.5
(a–d) Echocardiographic study in a 33-year-old patient with myocarditis and severe heart failure. Parasternal long-axis view (a) and M-mode (b) show severe left ventricular (LV) dilation (end-diastolic diameter corrected for body surface area 39 mm/m2), normal wall thickness, and severe systolic dysfunction (fractional shortening 12 %, ejection fraction 15 %). M-mode recorded at the level of the mitral valve (c) shows a B bump (arrow), a sign of elevated LV diastolic pressure. Color-Doppler imaging reveals severe functional mitral regurgitation (d)

Fig. 21.6
(a–c) Echocardiogram in a patient with acute myocarditis and severe left ventricular (LV) dysfunction showing multiple pedunculated and mobile thrombi in the LV cavity. Apical four-chamber view (a) demonstrating thrombus in the LV attached to the anterior mitral annulus. Apical long-axis view (b) showing another pedunculated mobile thrombus attached to the septal apex. Parasternal short-axis view (c) in which the apical thrombus is clearly visible
Recent studies show that LV strain and strain rate may be promising diagnostic parameters in acute myocarditis, even in the presence of normal standard indices, such as LV EF and wall motion. Di Bella et al. [79] demonstrated that strain Doppler echocardiography could detect longitudinal segmental myocardial dysfunction subsequent to myocardial edema in the acute phase of myocarditis.
Further studies using 2D speckle-tracking strain imaging demonstrate that, among all deformation parameters, longitudinal strain appears to be the most powerful index for diagnostic purposes [80, 81]. Furthermore, Escher et al. [82] demonstrated reduced 2D speckle-tracking global systolic longitudinal strain and strain rate in all their patients with biopsy-proven myocarditis (even in those who had preserved LV systolic function); in addition, at follow-up, deformation parameters were significantly lower in patients with than in those without inflammation and correlated significantly with lymphocytic infiltrates. Finally, in a preliminary report, myocardial contrast echocardiography enabled detection of regional perfusion defects that did not conform to a coronary distribution and were not apparent with conventional echocardiography, suggesting the diagnosis of myocarditis later confirmed by CMR [83].
CMR is extensively assessed in scientific literature and is frequently used in clinical practice to evaluate suspected myocarditis [84]. Acute myocarditis, particularly if presenting as acute chest pain and positive markers of myocardial damage without coronary artery disease, frequently has a characteristic pattern at LGE imaging of subepicardial enhancement involving the basal and mid segments of the LV free wall [85] (Fig. 21.7). T2- and T1-weighed early enhancement sequences may, respectively, demonstrate localized myocardial edema and global hyperemia in the inflamed myocardium [86, 87] (Fig. 21.7). The diagnostic accuracy of CMR compared with the gold-standard of EMB is very high when using a combination of two of the three aforementioned criteria [88].
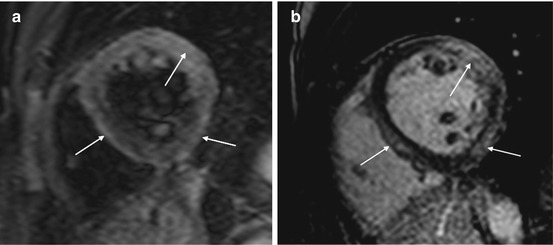

Fig. 21.7
(a, b) Cardiac magnetic resonance study in acute myocarditis. Midventricular short-axis T2-weighed image (a) showing multiple foci of myocardial edema (arrows). Midventricular short-axis T1-weighted image after gadolinium contrast administration (b) showing late gadolinium enhancement (transmural or subepicardial) in regions corresponding to hyperintensity seen at T2 imaging (arrows)
The same diagnostic criteria were initially reported to have high diagnostic accuracy in cases of myocarditis with clinical presentation such as HF [89]. However, more recent data report limited accuracy when evaluating a single criterion or any combination thereof [90, 91]. These cases are usually characterized by lower-grade diffuse inflammation—which is often impossible to identify with early-enhancement T1- or T2-weighted sequences—and by diffuse interstitial fibrosis, which is missed by LGE sequences [92]. Delayed multidetector CT performed 5 min after contrast injection may be able to identify hyperenhancement similarly to LGE in CMR [93]. This may be a useful alternative in patients with contraindications to CMR.
Echocardiographic recognition of severe myocardial structural derangement with LV enlargement and systolic dysfunction permits identification of patients at risk of major clinical events (death, heart transplantation) in the long-term follow-up. More importantly, data published by our group show that improvement or normalization of LV function at 6 months echocardiographic re-evaluation are associated with a benign long-term prognosis, independently of the pattern of clinical presentation and LV function at presentation [70].
Promising data have been published in recent years on the prognostic role of CMR in the context of myocarditis. The presence of LGE is associated with increased global and arrhythmic cardiac mortality [94]. A pattern of LGE involving the septum, usually from human herpesvirus 6 (HHV6) or combined HHV6 parvovirus B-19 (PVB19) infections, may be predictive of persisting LV dilatation and dysfunction [95].
21.6 Chagas Disease
One peculiar form of myocarditis is Chagas disease, which is caused by Trypanosoma cruzi, a flagellated protozoan. This disease continues to represent a health threat for an estimated 28 million people, mostly those living in Latin America [96, 97]. Chagas disease classically presents in an acute or initial phase, which is followed by a chronic phase that can be categorized into indeterminate, cardiac, or digestive forms, each with different clinical manifestations [97]. Severe acute disease is rare (<1 %) and characterized by acute myocarditis, pericardial effusion, and/or meningoencephalitis. After the acute phase, an indeterminate phase usually lasting several years precedes the chronic phase of the disease. The most common and serious problems are cardiac and are caused by an inflammatory CMP as a result of immune reaction and/or the persistence of parasites in the heart. They manifest clinically with progressive HF, life-threatening arrhythmias, and/or thromboembolic events. According to severity of clinical, ECG, chest X-ray, and imaging abnormalities, chronic Chagas CMP is classified into four stages (A, B, C, D) with different mortality and morbidity rates [98]:
Stage A, or indeterminate, characterized by the absence of symptoms and ECG and structural cardiac abnormalities
Stage B, with ECG abnormalities (conduction defects and/or arrhythmias) in the absence of symptoms; mild/moderate LV WMA and systolic dysfunction may be present
Stage C, LV dysfunction at imaging, and prior or current symptoms of HF
Stage D: symptoms/signs of HF at rest, refractory to medical therapy [New York Heart Association (NYHA) IV]
Most patients affected by Chagas disease have no symptoms for decades after becoming infected with T. cruzi. In rare cases, acute myopericarditis can manifest at echocardiography as pericardial effusion, sometimes massive and with tamponade, and/or WMA [97, 99]. In chronic Chagas disease, echocardiographic abnormalities increase in frequency and severity according to the clinical stage of the disease [97, 99, 100]. The typical, although not pathognomonic, pattern is the presence of apical LV aneurysm, with or without global chamber dilatation and depressed systolic function (Clips 21.7a, 21.7b, 21.7c, and 21.7d) [99, 100]. Diastolic dysfunction is an important hallmark of Chagas disease and usually precedes systolic dysfunction [97]. Also, RV involvement is a typical feature of the disease and is usually associated with LV dysfunction; occasionally a RV apical aneurysm is observed [97]. Patients with LV aneurysms, similarly to those with this complication of myocardial infarction, are at risk of thrombosis and systemic embolism.
Few data are available regarding the usefulness of new echocardiographic techniques in Chagas disease. Tissue-Doppler-imaging (TDI) derived myocardial strain can demonstrate lower radial and longitudinal values compared with normal individuals and could quantify subtle segmental contractile dysfunction not detected visually [99]. Speckle-tracking technology is used to quantify global and segmental LV deformation (radial, circumferential, and longitudinal strain) and twist and untwisting velocities [101]. In that study, global strains showed a significant decreasing trend across groups of disease severity. Interestingly, patients in the indeterminate form had significantly lower radial strains in both the global and midinferior segment and lower twist and untwisting velocities compared with normal individuals. CMR may help in diagnosing Chagas CMP by demonstrating aneurysm formation with preferential sites at the apex and inferolateral walls. Aneurysms are easily detected with SSFP cine imaging. The pattern of LGE is variable and may involve any or all layers of the myocardial wall [102, 103].
Prognosis in Chagas disease mainly depends on the clinical stage of the disease [97]. No mortality is reported for patients in the indeterminate phase. In one series of 843 initially asymptomatic patients with Chagas disease [100], during long-term follow-up (mean 9.9 ± 5.3 years), a change in clinical stage, LV systolic dimension at M-mode echocardiography, and EF were the only independent predictors of mortality. The frequency and severity of echocardiographic abnormalities increases with increasing severity of disease stage. In that series, mortality and event rates were, respectively, 0 and 8 % of 505 patients in group 0; 1 and 26 % of 257 patients of group 1; and 14 and 52 % of 87 patients of group 2.
Nunes et al. [104] reported on the prognostic value of LA volume assessed by 2D echocardiography, adding incremental prognostic value to clinical factors (NYHA class), LV EF, RV function, and Doppler parameters of diastolic function [Ratio of transmitral E velocity to mitral annular E′ velocity (E/E′)]. In that series, an LA-indexed end-systolic volume >51 ml/m2 was associated with significant excess mortality. The prognostic impact of TDI index on LV diastolic dysfunction (E/E′) was subsequently analyzed by Nunes et al. [105], who showed a different and opposite prognostic significance of E/E′ according to LV systolic dysfunction severity. In fact, a high E/E′ (>15) was a strong adverse prognostic factor only for patients with mild to moderate LV EF depression (>30 %), whereas it was “protective” in the subset patients with severe LV systolic dysfunction. An interaction between RV dysfunction, frequent in the most severe cases with advanced congestive HF, was hypothesized.
21.7 Chemotherapy-Induced Cardiomyopathy
The incidence of complications after antineoplastic therapy is increasing in relation to the incidence of cancer and prolonged survival rate. Cardiotoxicity is one of the major complications. The Cardiac Review and Evaluation Committee established some criteria for diagnosing chemotherapy-related cardiac dysfunction [106], which consider clinical history, LV EF reduction, and presence of HF symptoms and signs. Echocardiography is recommended before the start of treatment and periodically during and after chemotherapy cycles. Anthracyclines (doxorubicin, daunomycin, idarubicin) are the most frequent chemotherapeutic agents involved in cardiotoxicity; however, adverse cardiotoxic effects are reported also for other drugs (mitoxantrone, 5-fluorouracil, cyclophosphamide, trastuzumab). Anthracycline may exhibit two different types of cardiac toxicity over time: early-onset cardiotoxicity (often expressed with ventricular or supraventricular arrhythmias), or late-onset chronic cardiotoxicity that emerges many years after chemotherapy has been completed. The risk and severity of anthracycline CMP is typically dose related. Clinical manifestations are those of severe biventricular HF with decreased EF and frequently severe diastolic dysfunction. Because of the progressive morphologic changes and the persistence of changes over a long period, symptoms are reported to occur any time up to months after stopping the drug. Although HF is reported to have a high mortality rate, successful treatment is possible [107].
Traditional therapies, such as anthracyclines, have been recognized for years as causing cardiovascular complications. Less expected were the cardiovascular effects of targeted cancer therapies, which were initially thought to be specific to cancer cells and would spare any adverse effects on the heart. In patients with breast cancer treated with anthracyclines, taxanes, and trastuzumab, systolic longitudinal myocardial strain and ultrasensitive troponin I measured at the completion of anthracycline therapy are useful for predicting subsequent cardiotoxicity and may help guide treatment [108].
Echocardiographic characteristics of anthracyclines-induced CMP are indistinguishable from other causes of LV dysfunction [109]. A typical pattern is a mildly dilated but severely dysfunctioning LV with severe diastolic dysfunction [restrictive filling pattern (RFP)}. RV dysfunction, MR, tricuspid regurgitation (TR), and pulmonary hypertension are frequently present (Fig. 21.8, Clips 21.8a, 21.8b, 21.8c, and 21.8d). Other antineoplastic drugs, such as the monoclonal antibody trastuzumab, can induce reversible forms of cardiac dysfunction [110].
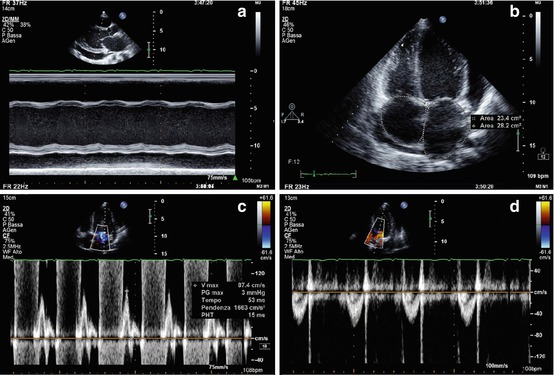

Fig. 21.8
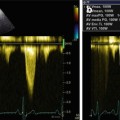
(a–d) A 19-year-old patient with cardiomyopathy that developed after chemiotherapy (including anthracyclines) and radiotherapy for acute lymphatic leukemia. M-mode echocardiography (a), parasternal long-axis view, shows moderate left ventricular (LV) dilation (end-diastolic diameter corrected for body surface area 36 mm/mq), with severe systolic dysfunction (fractional shortening 11 %). There is also pericardial effusion posteriorly to the LV (21-mm thick), around the right atrium (12 mm), and laterally to the LV (14 mm), as shown in the apical four-chamber view (b), which also shows mild biatrial dilation. Pulsed-wave-Doppler interrogation of transmitral flow reveals a restrictive filling pattern (c). Pulsed-wave Doppler of the aortic valve (d) shows a low velocity (0.5 m/s), a sign of low cardiac output
Furthermore, several forms of cancer treatment, such as 5-fluorouracil, are associated with an increased risk of coronary artery disease and/or acute coronary syndrome, with transient regional hypokinesia at echocardiographic evaluation.
Strain and strain-rate imaging is used to detect subtle early impairment in cardiac contractility due to chemotherapeutic agents. Reduced strain and strain-rate parameters can, in fact, precede any appreciable change in LV EF and might therefore help in identifying patients who develop chemotherapy-related cardiotoxicity in an early subclinical stage (Fig. 21.9) [111]. Early subclinical abnormalities occur after low to moderate dosages of anthracycline-based chemotherapy and persist after 6 months, although without evidence of myocardial fibrosis by LGE [112].
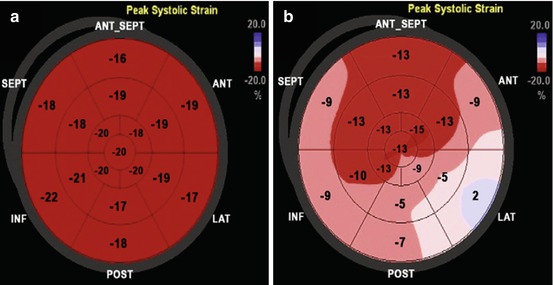

Fig. 21.9
(a, b) Normal 2D global longitudinal strain (−19.5 %) (a) measured in a 22-year-old woman before chemotherapy treatment. After 6 months of chemotherapy (b), a significant reduction in global longitudinal strain is observed (−10.1 %) despite persistence of normal left ventricular ejection fraction. ANT anterior, ANT_SEPT anteroseptal, INF inferior, LAT lateral, POST posterior, SEPT septal (From Oreto et al. [111], with permission)
CMR is able to detect significantly reduced LV EF and cardiac mass in survivors of childhood cancer previously undiagnosed with cardiotoxicity by 2D echocardiography [113, 114]. Indexed LV mass by CMR is inversely associated with cumulative anthracycline dose and predictive of events at follow-up [115]; LGE is rare [114, 115]. Diffuse myocardial fibrosis seen at T1 mapping may represent an early marker of anthracycline cardiotoxicity [116, 117].
Conversely, when trastuzumab is combined with anthracyclines for human epidermal growth factor receptor-2 (HER-2)-positive breast cancer, subepicardial lateral wall LGE is often found in patients who develop drug-induced CMP [118]. Serial LV EF determination may be performed using 99mTc radionuclide ventriculography and has long been regarded as the gold standard for measuring anthracycline cardiotoxicity in adult patients [119]. Imaging with [123I]-MIBG or 123I-labelled antimyosin may detect subclinical changes before LV function is impaired in patients treated with anthracyclines [120].
Regular heart-function monitoring during treatment is important to detect cardiac involvement in neoplastic patients treated with chemotherapy. A baseline, LV EF evaluation is usually necessary. It is recommended that the same methodology be used for comparing serial studies [109]. Decreased longitudinal strain, together with troponin I, increases early after treatment with anthracyclines and trastuzumab, can predict cardiotoxicity development [108]. CMR imaging is also emerging as a promising tool in the oncology setting [113].
A clinically important deterioration in LV function can be variously defined (i.e., 10 % absolute decrease in LV fractional shortening, 10 % decrease in LV EF, or absolute LV EF value <55 %). Guidelines recommend discontinuing anthracycline therapy if LV deterioration is found and, preferably, is confirmed on two successive tests [110].
Treating anthracycline-induced LV dysfunction is the same as for treating other causes of myocardial dysfunction according to international HF guidelines. Echocardiography continues to be the mainstay of monitoring therapeutic efficacy, given that it is portable, widely available, noninvasive, causes minimal pain, and provides real-time data [110].
21.8 Left Ventricular Noncompaction
A position statement from the European Society of Cardiology categorized LV NC as an “unclassified CMP,” asserting: “it is not clear whether it is a separate cardiomyopathy or merely a morphological trait shared by many phenotypically distinct cardiomyopathies” [121




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree