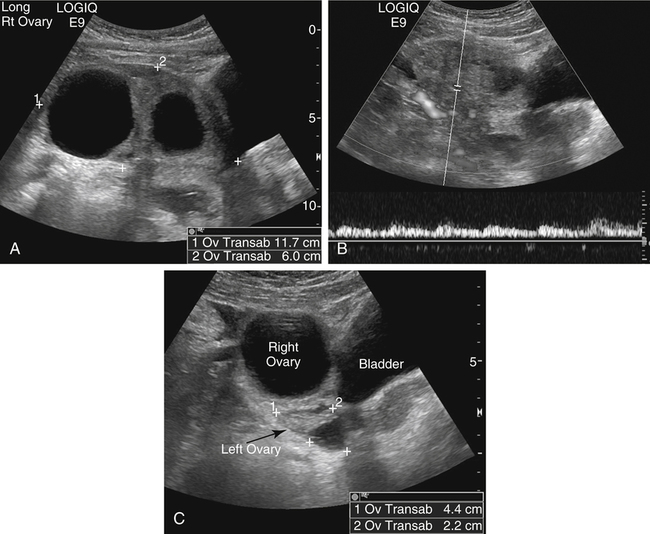
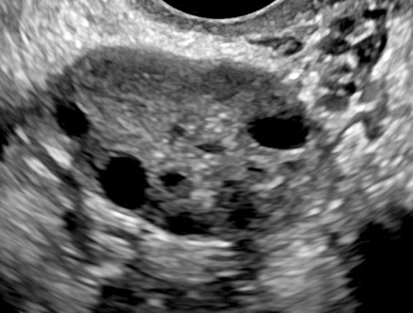
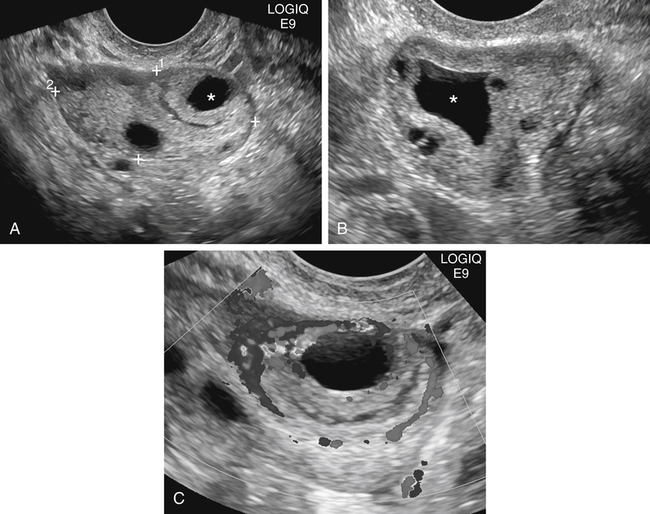
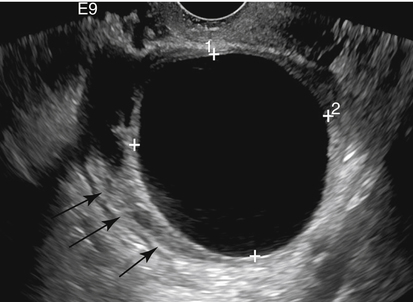
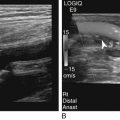
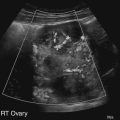
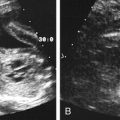
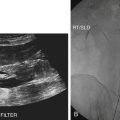
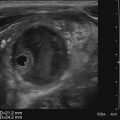
Diane J. Youngs and Douglas L. Brown Ovarian cancer usually produces nonspecific symptoms. In more than 70% of patients, malignant ovarian disease is diagnosed during the later stages, which significantly reduces the survival rate.1 Although early detection may improve survival, reliable ovarian cancer screening for the general population has not yet been developed. The most widely used serum tumor marker for epithelial ovarian cancer is cancer antigen 125 (CA 125). If a woman’s serum concentration of CA 125 is elevated, transvaginal sonography may be performed to assess for an ovarian mass. Only 50% to 60% of women with stage 1 epithelial ovarian cancer have elevated serum levels, and other benign conditions can elevate serum concentrations of CA 125 as well.2 The resultant low sensitivity and specificity limit the value of this test in detecting ovarian cancer. A combination of CA 125 testing with transvaginal sonography is sometimes performed as a screening method in women at high risk of having ovarian cancer.2 Despite the poor prognosis in most patients diagnosed with ovarian cancer, the lack of complete understanding of the pathogenesis of ovarian cancer, the increasingly recognized occurrence of interval cancers (cancers detected within a year after a negative screening test), and the high proportion of ovarian masses that are benign all make screening difficult. Multicenter trials are ongoing, and the results of these trials should provide useful information to determine whether screening is beneficial. Screening is not recommended for the general population at the present time.3 Women with a higher risk because of family history or known BRCA1 or BRCA2 (breast cancer gene) mutations may request sonographic screening, but even in these women, no benefit from screening has yet been shown.3 Although the ovaries are attached to the uterus by the ovarian ligament and to the infundibulum of the fallopian tube and pelvic side wall by the suspensory ligament, the ovaries are relatively mobile organs that typically reside in the adnexa anterior to the internal iliac vessels and medial to the external iliac vessels. Ovarian size varies with a woman’s menstrual status and age; mean ovarian volume is approximately 3.0 cm3 in premenarchal girls, 10 cm3 in menarchal women, and 6.0 cm3 in postmenopausal women.4 The ovaries are homogeneous in texture, with an echogenicity similar to that of the uterus and hypoechoic to surrounding bowel (Fig. 17-2). Anechoic follicles may be seen in the ovarian periphery, and the vascular medulla may appear echogenic compared with the cortex. The dominant follicle typically reaches 2.0 to 2.5 cm in size; however, it can reach 2.8 cm. Simple cysts seen within the ovary that measure less than 3.0 cm are assumed to be ovarian follicles. The corpus luteum forms from the ruptured dominant follicle, and luteinization is made possible by the development of numerous capillaries and a rich blood supply. Hemorrhage into the corpus luteum is very common, resulting in a hemorrhagic corpus luteum. The corpus luteum remains intact until about week 12 of a pregnancy or until day 23 of the menstrual cycle if pregnancy does not occur. The sonographic appearance of a corpus luteum is variable, but the corpus luteum is often irregular in shape with a thick wall that may be crenulated (Fig. 17-3, A and B). The corpus luteum is often highly vascular with abundant flow evident on color Doppler imaging (Fig. 17-3, C; see Color Plate 13). It is a normal structure and should not be misinterpreted as pathology. After menopause, the ovaries atrophy and are frequently devoid of follicles, which make them difficult to identify. Postmenopausal women may occasionally have small cysts or residual follicles; generally, a simple cyst less than 1 cm can be ignored in postmenopausal women.5 When an adnexal mass is discovered during sonographic evaluation, the location, echotexture, size of the mass, and any associated findings such as ascites should be documented. Determining whether a mass is cystic, solid, or complex (a nonspecific term generally used to denote a combination of cystic and solid components) requires observation of the following: acoustic enhancement or attenuation, the presence of internal echoes by optimized gain and frequency, loculation, thickness and regularity of any septations, and whether solid components represent tissue versus blood or debris, which can be determined by applying color or power Doppler imaging. One also needs to consider nonovarian causes for findings such as tuboovarian abscess, pyosalpinx or hydrosalpinx (see Chapter 15), ectopic pregnancy (see Chapter 21), or pedunculated fibroid (see Chapter 13). Many benign ovarian masses do not demonstrate sonographic evidence of vascularity in the internal components by Doppler imaging. Color Doppler imaging demonstrates peripheral flow around a simple cyst, a hemorrhagic cyst, an endometrioma, or cystic teratoma, but vascularity is not evident within the internal components. When the solid-appearing components of an ovarian mass demonstrate vascularity on color Doppler imaging, a benign or malignant neoplastic process must be considered. Table 17-1 presents sonographic characteristics associated with benign and malignant ovarian masses. TABLE 17-1 Benign and Malignant Characteristics of Ovarian Masses If an ovarian follicle or corpus luteum fails to regress, it can continue to fill with fluid and result in a functional or physiologic cyst. The term “functional cyst” means that the cyst is ovarian in origin and responds to cyclic hormonal changes. The functional ovarian cysts discussed in this chapter are follicular cysts and hemorrhagic cysts. Theca lutein cysts are functional cysts related to human chorionic gonadotropin exposure and are discussed in Chapter 16. Follicular cysts are one of the most common causes of ovarian enlargement in young women. They occur when a dominant follicle fails either to ovulate or to regress. Anechoic cystic spaces within the ovary are assumed to be follicles and are referred to as a “cyst” only when they measure greater than 3.0 cm. The walls of follicular cysts are smooth and thin. Follicular cysts typically range in size from 3.0 to 8.0 cm but may attain larger diameters. They are usually asymptomatic but may produce dull adnexal pressure and pain. In most cases, these cysts eventually rupture or are resorbed. In premenopausal women who are or become asymptomatic, follow-up sonography is generally suggested if the cyst is larger than 5.0 cm.5 Although the ovaries cease to function during menopause, it is not unusual to identify ovarian cysts in a postmenopausal patient. MRI or surgical evaluation is generally recommended only if an ovarian cyst measures more than 7.0 cm in premenopausal and postmenopausal patients.5 Follicular cysts most commonly appear as anechoic, thin-walled unilocular structures demonstrating acoustic enhancement (i.e., as simple cysts). Occasionally, these cysts may contain diffuse, low-level echoes that reflect bleeding into the cyst. Ovarian tissue can be seen around the periphery of the cyst (Fig. 17-4). If follow-up sonography is performed on a symptomatic patient, most follicular cysts resolve within about 2 months. Hemorrhage into a follicular cyst or excess hemorrhage into a corpus luteum may result in a hemorrhagic ovarian cyst. Most hemorrhagic cysts resolve within 8 weeks. However, as with follicular cysts, asymptomatic women with cysts less than 5.0 cm probably do not need routine sonographic follow-up.5 Hemorrhage within the cyst creates predictable patterns described as the “fishnet” or reticular pattern (Fig. 17-5, A) or retracting clot (Fig. 17-5, B). Hemorrhagic cysts may also demonstrate internal echoes with a fluid/fluid level (Fig. 17-5, C). Sometimes a hemorrhagic cyst has a predominantly solid appearance, but color Doppler sonography demonstrates no blood flow within the cyst and may show a highly vascular rim around the periphery of the cyst (Fig. 17-6; see Color Plate 14). Most paraovarian cysts appear as a small, simple cyst separate from the ovary (Fig. 17-7). If the cyst is close to the ovary, it may be difficult to differentiate it from a follicular cyst. Application of gentle hand or transducer pressure may help separate the cystic structure from the ovary. Because there is no rim of ovarian tissue, large paraovarian cysts may resemble the urinary bladder. Differentiation from the bladder can be made by having the patient void. Because paraovarian cysts do not respond to the hormone cycle, follow-up examination is likely to demonstrate little or no change in size and appearance. Peritoneal inclusion cysts appear as a multiloculated cystic mass either adjacent to or surrounding an ovary (Fig. 17-8). The ovary may be in the center of the septations and fluid or peripherally located. The fluid is usually anechoic, but echoes may be evident if hemorrhage or protein is present.
Ovarian Mass
Normal Ovarian Anatomy and Physiology
Anatomy
Normal Sonographic Appearance
Key Sonographic Observations
Benign Characteristics
Malignant Characteristics
Purely cystic
Complex with thick septations
Findings consistent with hemorrhagic cyst, endometrioma, or cystic teratoma
Complex with mural nodules or other solid areas not typical of a dermoid
No internal flow by Doppler imaging
Ascites (more than normal small amount often seen in premenopausal women)
Complex mass is thin walled, has thin septations, no papillary projections or other solid areas
Solid components of mass demonstrate vascularity on color Doppler imaging
Benign solid masses tend to demonstrate mild to no vascularity
Functional or Physiologic Ovarian Cysts
Follicular Cyst
Sonographic Findings
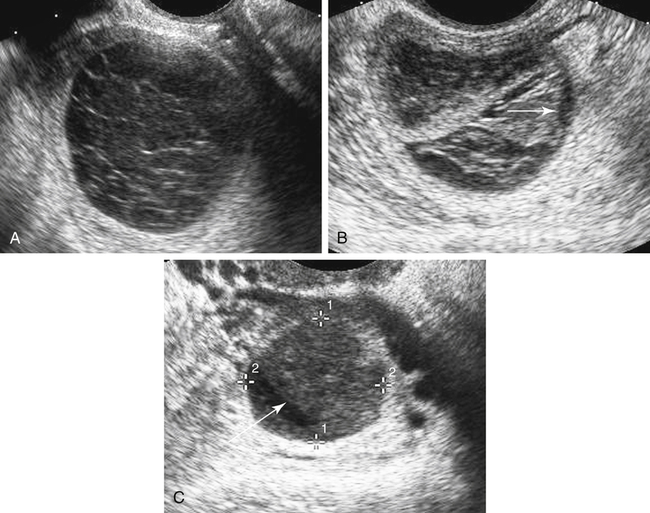
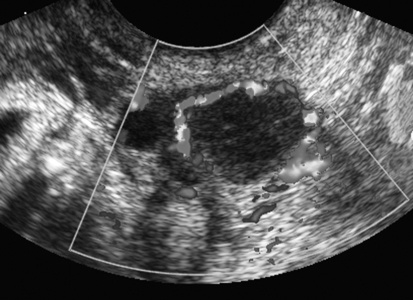
Hemorrhagic Cyst
Sonographic Findings
Nonfunctional Cysts
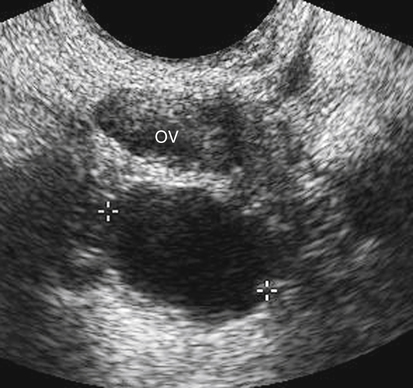
Paraovarian Cyst
Sonographic Findings
Peritoneal Inclusion Cyst
Sonographic Findings
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Ovarian Mass