Fig. 6.1
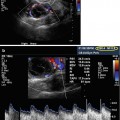
Decreased ovarian reserve with AFC of 3
Standard AFC assessment was, and still is, performed primarily with 2D US imaging. Although this modality might be sufficient in some cases, there might be some uncertainties and disadvantages.
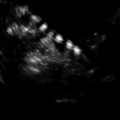
3D AFC is more reproducible and accurate but the method is less standardized, and 3D technology may not be freely available for all reproductive endocrinologists. Figure 6.2a, b shows 3D antral follicle count. The 3D count can be performed in inverse mode as shown (Fig. 6.2a). It should be noted that it is imperative to distinguish between the total antral follicle count (TAFC), including follicles >6 mm in diameter; however, the number of small antral follicles is more predictive of number of oocytes retrieved. AFC is also a predictor of pregnancy loss and low AFC correlates with 4× increase in early miscarriages [9].
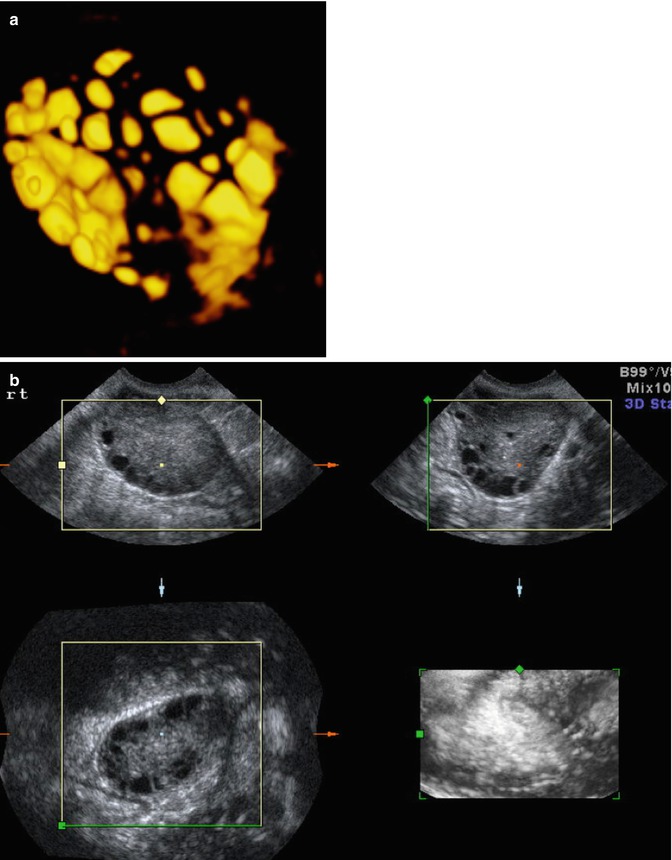

Fig. 6.2
(a) 3D AFC in inverse mode (b) Multiplanar view of ovary with antral follicle count
Endocrine Markers of Ovarian Reserve
Endocrine markers of ovarian reserve are anti-müllerian hormone (AMH) and inhibin B which are direct markers of quantity and follicular cohort numbers. Indirect markers are basal day 3 FSH and estradiol (E2) levels [10–16], and high levels indicate a decreased number of small antral follicles. Both AFC and AMH predict similarly the response to treatment, but ultrasound is the only method so far that allows a direct assessment of each ovary separately. Identification of participants who are likely to respond poorly during IVF treatment is clinically relevant as the couple can be counseled regarding cycle cancellation and lower chance of success. Pretreatment AFC and AMH were found to be the most significant predictors of the number of oocytes retrieved especially for low and high responders in multiple studies including a meta-analysis by Hendricks et al. [2, 10–16]. These studies showed that AFC and AMH demonstrated similar predictive power based on ROC area under the curve (AUC) analysis and correlated with oocyte number better than other parameters such as FSH or age. However, all these parameters correlate less well with pregnancy outcomes, which is a more important outcome for the patient than oocyte number. In addition, AMH and AFC have been shown to be the best predictors of OHSS in women undergoing ovarian stimulation for IVF. The advantage of AMH is that it is not operator or cycle day dependent. A cutoff level of >3.3 ng/mL determines the risk of OHSS with a 90 % sensitivity,71 % specificity, and 61 % PPV, and a cutoff value of AFC >8 predicts the risk of OHSS with a 78 % sensitivity, 65 % specificity, and 53 % PPV [17].
The validity of AFC for ovarian reserve comes from studies showing a direct correlation with the number of nongrowing follicles viewed on histologic sections [18]. On the other hand, ovarian volume, vascularity, and perfusion had no significant value in predicting poor ovarian response and all are inferior to AFC [19]. The hypothesis that aneuploidy is negatively associated with the quantity of oocytes in the ovary is supported by studies showing decrease AFC in women with spontaneous abortions after IVF. The conclusions are not supported by all studies possibly because some lack power and it may depend on the mechanism of diminished ovarian reserve. In many women with low AFC, especially at a young age, there is a decrease in quantity but not in quality of the oocytes.
3D Ultrasound and Ovarian Volume
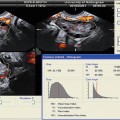
Ovarian volume can be calculated by measuring each ovary in three perpendicular directions and applying the formula of the ellipsoid (D1 × D2 × D3 × π/6). Ovarian volume can also be automatically calculated using the software called “virtual organ computer-aided analysis” or VOCAL (Fig. 6.3). This imaging program calculates organ volume from the areas of the three orthogonal sections, sagittal, transverse, and coronal views, and allows very precise calculation of ovarian volumes. However, ovarian volume can be affected by ovarian cysts. Ovarian volume and antral follicle volume can now be automated. Three-dimensional US is an excellent technique for calculating ovarian volume very precisely using the VOCAL program and observing the ovary with rotating angles. Low ovarian reserve and poor response to controlled ovarian hyperstimulation in ART are associated with volumes <3 cm3 as seen by Lass and colleagues with increased cancellation rates [20]. Polycystic ovaries are associated with volumes >6.6 cm3. Ovarian hyperstimulation syndrome ( OHSS) is associated with increased ovarian volume [21, 22]. However, total volume of the ovaries detected by transvaginal ultrasound is not better than the AFC in predicting parameters.
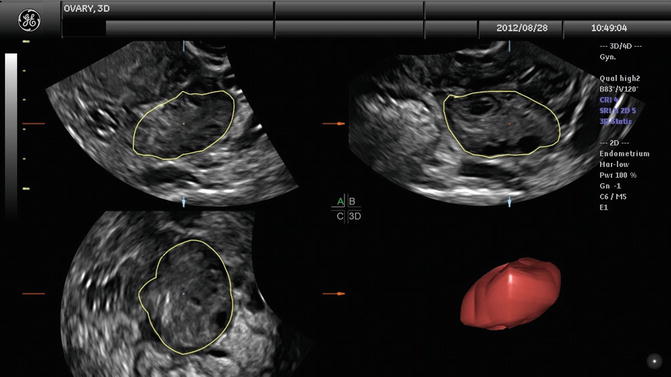

Fig. 6.3
Ovarian volume with VOCAL program. (a–c) Multiplaner view of ovary with ovarian volume
The studies of IVF patients have demonstrated that 3D ultrasound volume measurements for follicles correlate better with the volume of aspirated follicular fluid than 2D ultrasound measurements [22]. One of the most frequently employed applications is the sonography-based Automated Volume Calculation (SONO-AVC; GE Medical Systems, Zipf, Austria). The application of SonoAVC for IVF was first described by Raine-Fenning et al. [23]. Studies with SonoAVC have not shown a clear benefit in improving IVF outcomes [24]. Even if there is no clinical benefit, the advantages of SonoAVC may be a time decrease during the ultrasound as the ovarian volumes are saved and can be calculated later. This may lead to less discomfort for the patients. However, there is time required for manual assessment of the 3D data which should be added to the time in scanning and the technique needs to be learned and reproducibility documented. Rodriguez Fuentes analyzed the impact of SonoAVC on time and the clinical outcome of IVF treatment. They found reduced time saving of 4 min per case after including the post-processing time [24]. Their study has shown that SonoAVC provides different results from those of 2D ultrasound imaging when the size of the follicle is considered.
Evaluation of Ovarian Stroma Flow with 3D Ultrasound
It is possible that poor ovarian vascularization impairs access of gonadotropins to the ovarian follicles. Power Doppler US in combination with 3D US and VOCAL is a very good approach for correlating the ovarian vascular network with the ovarian response to ART. The significance of ovarian stromal blood flow with ovarian reserve was studied [19]. Variably, some studies show correlation of undetectable basal ovarian stromal blood flow with poor response and others did not.
Ovarian Cysts and Masses
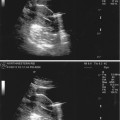
Ovarian cysts are important to diagnose by ultrasound in the infertile patient and can affect the ovarian volume. In addition, ovarian cysts and masses can be picked up on baseline ultrasounds for ovarian reserve. The most common ovarian cysts seen in infertility patients are simple functional cysts, hemorrhagic cysts, endometriomas, and dermoid cysts. Studies verify that evaluation of ovarian masses by basic ultrasound based on morphology is equal to other methods with a sensitivity of 88–100 % and a specificity of 62–96 % for predicting malignancy [25]. The majority of ovarian masses that will be seen on the baseline ultrasound will be one of the big 6. This includes functional cysts or follicles, corpus luteum cyst, hemorrhagic cysts, endometriomas polycystic ovaries, and benign cystic teratomas (dermoids). Functional cysts are the most common cystic masses seen on ultrasound in the reproductive age group. These cysts tend to stay less than 10 cm and regress after 1–2 cycles and are either follicular cysts or luteal cysts. A functional follicle can develop when ovulation fails to occur and is simple in ultrasound appearance (Fig. 6.4). If they are small (<3 cm) and not hormonally active, they do not need to be treated before ART. However, patients with large simple ovarian cysts may have lower response to stimulation, and ovarian cyst aspiration immediately prior to ovarian stimulation has been shown to be beneficial [26]. A corpus luteum cyst is more solid in appearance, and Doppler US demonstrates prominent peripheral blood flow with a low-resistance waveform (Fig. 6.5). Typically this cyst can resolve in a few weeks and is asymptomatic but can grow and cause pain, rupture, or torsion. A hemorrhagic cyst can develop from hemorrhage into a corpus luteum. Acutely there is clot and the clot may retract and resolve. It will jiggle with the movement of the transducer and fibrin strands are seen (Fig. 6.6).
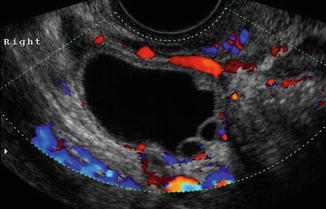
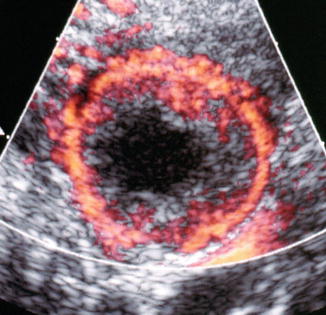
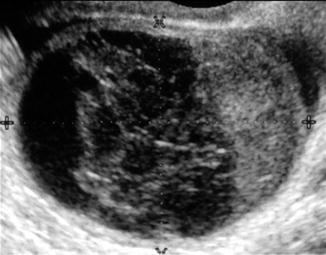

Fig. 6.4
Follicular cyst

Fig. 6.5
Corpus luteum (with ring of fire)

Fig. 6.6
Resolving hemorrhagic cyst
An endometrioma is also a very common finding in the infertile patient and is a sign of the presence of endometriosis in other areas [27, 28]. The typical endometrioma is a unilocular cyst with homogeneous low-level internal echogenicity (ground glass echogenicity) of the cyst fluid (Fig. 6.7). With respect to endometrioma, a correct diagnosis is important for infertility with possible need for ART. Transvaginal ultrasonography is the imaging of choice to differentiate ovarian endometriomas from other adnexal masses [29, 30]. Studies show that an endometrioma is associated with lower response to ovarian stimulation; however, removing the endometrioma can also affect ovarian response and may significantly diminish ovarian reserve as the base of the ovary is usually burned and the cyst wall is stripped with damage of follicles [31]. The trend has become to stimulate with the endometriomas present unless symptomatic and less surgery is necessary to enhance fertility [32].
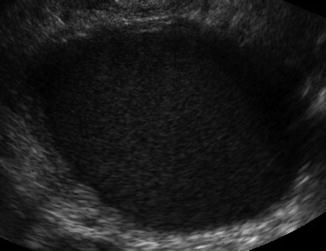

Fig. 6.7
Endometrioma
Polycystic ovaries are covered below. Dermoid cysts can present as solid hyperechoic heterogeneous masses with a mixed pattern of solid and cystic areas. They may contain calcifications, fat, and hair. The hair can disperse in the surrounding fluid as seen in Fig. 6.8. If the “hair ball” floats in the sebum, there is an appearance of a tip of the iceberg sign (Fig. 6.9). They should be removed prior to IVF if they are causing pain or if there is a question of malignancy. Puncture during oocyte retrieval should be avoided due to high risk of peritonitis.
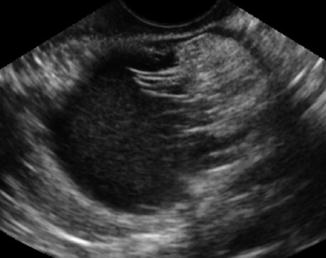
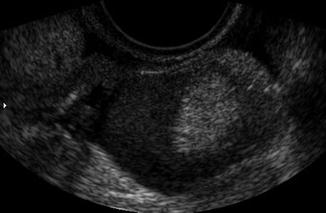

Fig. 6.8
Dermoid cyst with hair

Fig. 6.9
Dermoid cyst “tip of the iceberg” sign
Other extraovarian lesions include hydrosalpinges, paraovarian cysts, and peritoneal inclusion cysts that may be picked up on baseline ultrasound. These are benign and will be covered in other chapters. Features that are worrisome for primary epithelial malignancy include cysts with thick septations or vascularized areas of focal wall thickening, solid masses, and ascites (Fig. 6.10).
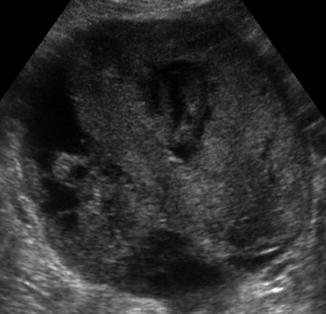

Fig. 6.10
Ovarian cancer
Ultrasound and Polycystic Ovary (PCO)
Polycystic ovary syndrome (PCOS) is one of the most common endocrine disorders impairing female fertility; it has been reported to occur in about 20 % of the general female population and in up to 50 % of women undergoing IVF therapy [33, 34]. It is covered in detail in another chapter but will be discussed briefly here. Ultrasound is one of the criteria for the diagnosis of polycystic ovarian syndrome (PCOS) based on the Rotterdam consensus conference. Current data suggest that polycystic ovaries detected by transvaginal ultrasonography may be found in approximately 75 % of women with a clinical diagnosis of PCOS [35, 36]. However, it is not a rule that all women with polycystic ovaries will demonstrate the clinical and biochemical features of PCOS, oligomenorrhea, and/or hyperandrogenism. Polycystic ovaries per se, even without PCOS, constitute a risk factor for the development of ovarian hyperstimulation syndrome (OHSS) and the stimulation protocol chosen should reflect this [37]. AMH is also an excellent marker for PCOS and OHSS.
Transvaginal ultrasound is a highly sensitive method for identification of PCO. The most commonly used criteria today are those proposed by Dewailly and colleagues with a string of pearls pattern [38, 39] where the antral follicles are arranged peripherally with a dense core of ovarian stroma (Fig. 6.11). This was reaffirmed in the Rotterdam 2003 consensus [40]. The transvaginal definition is based on the presence of an ovarian volume of greater than 10 cm3 (milliliters) >12 small follicles in a single ovary. Comparisons between transabdominal and transvaginal ultrasound do not find significant differences in the detection rate of PCO. The 3D ultrasound and the use of color and pulsed Doppler ultrasound showing increased ovarian blood flow are techniques which further enable the identification of PCO, but are not mandatory for the diagnosis [39]. No other imaging modality, such as magnetic resonance imaging (MRI) for the visualization of the ovaries, are needed for the diagnosis of PCO and should not be used use as routine examination.
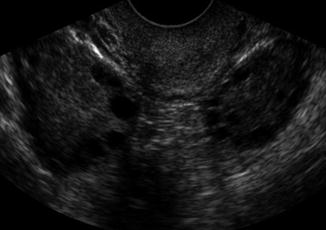

Fig. 6.11
PCOS
The new automated 3D US techniques facilitate the exclusion of a false-positive PCOS diagnosis and reflect pathophysiological changes in these patients in a more accurate manner. An early detection of PCOS is highly recommended for women undergoing IVF treatment due to the elevated risk for OHSS. Unfortunately, because 3D ultrasound is a relatively new imaging modality, the Rotterdam criteria only take 2D US sonography into account.
At present, only a few studies have evaluated the use of 3D US for PCOS patients [41, 42]; furthermore, to date, only one study has addressed automated 3D US (SonoAVC) [42]. In a retrospective cohort study, Allemand et al. [41




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree