Oxidative Metabolism and Cardiac Efficiency
Heikki Ukkonen
Rob S.B. Beanlands
Oxidative metabolism of fuels provides the heart with the energy required for contraction and basal metabolism. Impaired myocardial energy transfer plays an important role in the development of contractile dysfunction in many clinical syndromes, including ischemic heart disease. Positron emission tomography (PET) provides a unique tool for noninvasive quantitative characterization of myocardial metabolic processes in vivo, and it has been widely used for the detection of viable myocardium in ischemic heart disease. However, the capabilities of PET to define myocardial metabolism have not been used to their fullest extent. Currently, the efficacy of therapy is monitored primarily by evaluating patients’ functional capacity, ventricular function, and hemodynamics. By combining the functional data with PET-derived metabolic data, the treatment effects can be more physiologically assessed. This approach has been applied to measure myocardial efficiency (i.e., myocardial work related to myocardial oxygen consumption) to evaluate cardiac disease and its therapies.
This chapter focuses on myocardial metabolism, particularly the application of oxidative metabolism and oxygen consumption imaging with PET, in cardiac disease and treatment. The role of PET in the determination of myocardial efficiency is also emphasized; application of this methodology in the optimization of therapy is expected to increase as PET becomes more widely available.
Myocardial Metabolism
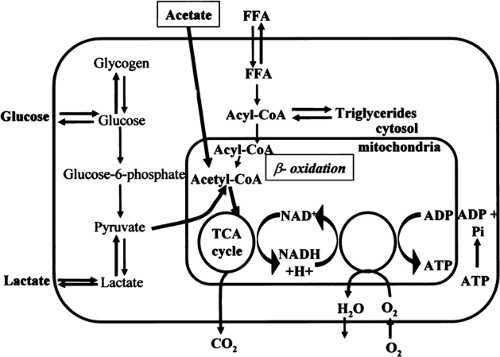
The heart requires a constant supply of energy to sustain contractile function. This energy is supplied by hydrolysis of adenosine triphosphate (ATP), which is primarily derived from aerobic metabolism of fatty acids and carbohydrates and to a significantly lesser extent from aerobic metabolism of amino acids and ketone bodies (Fig. 11.3.1). Although the mechanisms that connect the mechanical work and energy production of the heart are not fully understood, it has been suggested that intracellular calcium plays a regulatory role in the link between cardiac mechanics and energy production (1).
Oxygen is the final electron acceptor in all pathways of aerobic metabolism in the myocardium. Therefore, under steady state conditions, myocardial oxygen consumption provides an accurate measure of overall myocardial metabolism (2). However, the relative utilization of different energy substrates depends mainly on the concentration of these substrates in the afferent blood vessels, on hormonal influences, workload, blood flow, and oxygen demand (3).
ATP is the immediate and quantitatively by far the most important substance that fuels most myocellular processes. Because the myocardium relies predominantly on aerobic metabolism for its energy requirements, myocardial oxygen consumption ultimately reflects the rate of mitochondrial metabolism and ATP production.
The processes of the heart requiring ATP (and therefore oxygen) may be divided into three main categories: basal metabolism, excitation–contraction coupling (ion movements against electrochemical gradients), and force generation by the actin and myosin molecules (4). In clinical terms, the major determinants of myocardial oxygen consumption are basal metabolism of the heart, heart rate, and myocardial wall stress and contractility. Wall stress and contractility are the principal components of “force generation” that results from the actin–myosin interaction.
Approximately 65% to 80% of the total energy produced is converted to heat and the rest is available for force generation and basal metabolism. Basal metabolic energy is required for protein synthesis and maintenance of cellular membrane integrity. The energy needed for excitation–contraction coupling, that is, energy for calcium cycling, is at most 20% to 25% of the total ATP consumption during the isovolumetric contraction phase (5,6).
Cross-bridge activation by actin and myosin molecules (myosin ATP), which leads to myocardial contraction, uses approximately 65% of the energy available for all mechanical processes of the heart.
Cross-bridge activation by actin and myosin molecules (myosin ATP), which leads to myocardial contraction, uses approximately 65% of the energy available for all mechanical processes of the heart.
Fatty Acid Oxidation
Free fatty acids (FFAs) are considered the preferred substrate for myocardial metabolism (7). In the fasted state and after a meal rich in fat, the level of blood FFAs is high and FFAs become the major source of energy, accounting for up to 90% of myocardial oxygen consumption (8). In addition, myocardial workload influences substrate metabolism. FFAs are preferred over glucose up to a moderate level of exercise (9,10). When fatty acids are oxidized, glucose oxidation is inhibited, and the glucose is shuttled into glycogen synthesis (3). Myocardial uptake of albumin-bound FFAs is related to the level of FFA in the blood and to the FFA:albumin ratio. Once inside the myocardial cell, fatty acids are activated to long-chain acyl-coenzyme A (acyl-CoA) by an acyl-CoA synthetase (Fig. 11.3.1). Long-chain acyl-CoA is oxidized to produce acetyl-coenzyme A (acetyl-CoA) (so-called β-oxidation). Each molecule of acetyl-CoA that is oxidized by β-oxidation produces one molecule of NADH (nicotinamide adenine dinucleotide, reduced) and one molecule of FADH2 (flavin adenine dinucleotide, reduced). Acetyl-CoA then enters the tricarboxylic acid (TCA) cycle and produces two molecules of carbon dioxide, three of NADH, and one of FADH2 for each molecule of acetyl-CoA (see later discussion).
Carbohydrate Oxidation (Glucose, Lactate)
Besides FFA, the other main source of acetyl-CoA for the TCA cycle is oxidation of carbohydrates, particularly glucose and lactate. When the organism is in a carbohydrate-fed state, lipolysis is inhibited by insulin and subsequently carbohydrate oxidation increases (11). In this case, carbohydrates can account for 100% of the myocardial oxygen consumption (glucose, 70%; lactate, 30%). During heavy dynamic exercise (65% of an individual’s maximal oxygen uptake), the production of lactate increases, and it becomes the major fuel of the heart, accounting for 60% to 70% of the myocardial oxygen consumption (3,12).
During ischemia, the metabolism of glucose to lactate is the main source of energy for the heart. With the lack of a sufficient oxygen supply, glucose uptake, glycogenolysis, glycolytic flux, and ATP hydrolysis are all stimulated (13).
Glucose is transported into the myocardial cell by the glucose transporters GLUT4 and GLUT1 (14). Intracellularly, glucose is rapidly phosphorylated to glucose 6-phosphate, which is further used for glycolysis or glycogen synthesis (Fig. 11.3.1). In glycolysis, glucose 6-phosphate is metabolized to pyruvate. Pyruvate is also formed from lactate taken up by the heart. Under aerobic conditions, most of the pyruvate is converted to acetyl-CoA, which enters the TCA cycle (see later discussion). A minor portion of glucose and lactate is converted to oxaloacetate, which enters the TCA cycle at a different site than acetyl-CoA (13).
Tricarboxylic Acid Cycle
The metabolic pathways for energy substrates transform the major fuels into acetyl-CoA, which then enters the TCA cycle. One molecule of acetyl-CoA produces three molecules of NADH, one of FADH2, and one of guanosine triphosphate (GTP), which is also a high-energy compound. NADH and FADH2 are oxidized in the respiratory electron transport chain and ultimately yield 11 molecules of ATP. Mitochondrial respiration appears to be regulated by nitrous oxide (NO), at least in vitro, and thus NO might have a regulatory role in the myocardial metabolic rate of oxygen (MMRO2) in vivo (15,16).
The rate at which the TCA cycle operates is the major factor that controls the rate of production of ATP by the heart. The TCA cycle activity increases when myocardial work increases. Reduced
ATP:adenosine diphosphate and NAD:NADH2 ratios may be the major determinants of the rate at which the TCA cycle operates (17), but this has been disputed (18).
ATP:adenosine diphosphate and NAD:NADH2 ratios may be the major determinants of the rate at which the TCA cycle operates (17), but this has been disputed (18).
Efficiency
In the healthy heart, myocardial metabolism and contraction are closely linked by ATP production and consumption. However, in certain clinical conditions, such as ischemic heart disease, in which oxygen supply is restricted, and in heart failure, this close connection can be lost. The overloaded failing heart is characterized by an imbalance between energy production and utilization (19). The overload itself increases energy expenditure by increasing wall stress in the dilated heart. The energy deficit of the myocardium may further aggravate both systolic and diastolic dysfunction (19).
Myocardial metabolism can be related to myocardial work to further evaluate cardiac physiology. The concept of efficiency has been used for decades for this purpose (20) and was originally described by Starling and Vissher (21) in isolated heart preparations. Efficiency is defined as the ratio between the energy created by a system and the energy put into the system. Because most of the energy (approximately 65% to 80%) consumed by the heart is converted to heat, experimental thermodynamic studies are important for assessing the total efficiency of the heart (22,23). However, in vivo (in animal and human studies), efficiency is usually defined as the ratio of mechanical work performed relative to the myocardial oxygen consumption (mechanical efficiency). It has been claimed that the efficiency is maximized under physiologic conditions both in the right ventricle (RV) and in the left ventricle (LV) (20,24). However, the question whether the heart is optimized to operate at maximum efficiency or at maximum stroke work is still a matter of controversy.
In 1979, Suga (25) introduced a comprehensive time varying elastase model. In this model, the area of the pressure–volume relationship (PVA) is a measure of the total work performed by the ventricle during one cardiac cycle. The PVA concept includes both the external or stroke (pressure–volume) work of the heart and an internal component, termed potential energy. The PVA correlates closely with invasively measured MMRO2 per beat (25). The method allows the assessment of contractile efficiency, as well as the cost of basal and activation metabolism. The PVA is the only one of the mechanical variables that can be used to assess myocardial efficiency without a need to separately measure myocardial oxygen consumption. The PVA method to measure oxygen consumption is highly invasive, requiring catheterization with LV micromanometers and conductance catheters. Therefore, the applicability of the method in human studies is limited.
Other approaches require a measure or estimate of both external work and oxygen consumption to determine efficiency. There are several applicable approaches for noninvasive measurement of external cardiac work, which usually employ measurement of blood pressure and heart rate with or without stroke volume (26,27). External work can be assessed with the pressure work index, a product of systolic (or mean) pressure, heart rate, and stroke volume (28), which covers both pressure and flow work. Energy is used for building and maintaining tension, which is needed for contractility and wall stress. Systolic contraction of the ventricle is enhanced by preload and is opposed by afterload, which are both oxygen-requiring processes (29). When related to MMRO2, the pressure work index gives a measure of useful forward mechanical efficiency. The pressure work index and its modifications are readily assessed noninvasively in vivo, which improves the applicability of this method in human studies (30,31,32,33,34).
Assessment of Myocardial Energy Metabolism
In Vitro Methods
A common way to study the energy metabolism of the heart is to measure the intracellular levels of metabolic intermediates or the activity of enzymes involved in the various pathways of energy metabolism. With these techniques, the levels of intermediates are measured from frozen or lyophilized tissue samples by spectrophotometry, radiometry, or high-performance liquid chromatography. Although these methods have formed the basis of our understanding of myocardial metabolism, they require tissue samples and are not clinically practical. These methods are also limited in that they can give relevant information about the energy status of the heart, but not information about the rate of the production or utilization of ATP (i.e., oxygen consumption) (35).
Invasive Methods
Percutaneous catheterization of the coronary sinus and blood sampling allow the measurement of cardiac metabolism in vivo. Simultaneous measurements of the concentration of energy substrates (carbohydrates, fatty acids) or oxygen in the coronary sinus and in the arterial blood and coronary blood flow allow calculation of net rates of carbohydrate and lipid metabolism and myocardial oxygen consumption (36). The net substrate metabolism can be calculated more accurately if specific substrates labeled with carbon-14 ([14C]) or tritium are used. Despite improved accuracy, regional myocardial metabolism cannot be assessed with such methods. Since coronary sinus catheterization is a highly invasive and sometimes difficult procedure, the use of this approach is limited.
Noninvasive Methods
Nuclear Magnetic Resonance
Nuclear magnetic resonance (NMR) spectroscopy allows assessment of molecular structure and substrate concentration. The two most common nuclides measured in studies involving energy metabolism are phosphate-31 ([31P]) and carbon-13 ([13C]). The use of [31P] NMR provides information about the levels of ATP, creatinine phosphate, inorganic phosphate, and sugar phosphates, all of which play a role in the myocardial energy system (35). Various intermediates of metabolism can be labeled with [13C]. NMR spectroscopy allows quantitative detection of these intermediates in the tissue, an approach that has been used to assess TCA cycle activity. Fluorine-19 ([19F])-labeled compounds that bind calcium have been used with NMR to measure intracellular calcium.
Phosphate-31 NMR provides information about the concentration of high-energy phosphate compounds, but not about the rate of ATP production or utilization, which limits the usefulness of this method. Carbon-13 NMR can be used to detect the fate of a predefined substrate in the chain of oxidative metabolism (TCA cycle) (37). However, TCA cycle intermediates and the [13C] label are quickly equilibrated, and as a consequence, [13C] NMR mainly measures incorporation of [13C] into the glutamate pool (37). Carbon-13 NMR is further limited by difficulties in kinetic analysis of metabolite labeling (35).
Magnetic Resonance Imaging
Recent technical advances in cardiac magnetic resonance imaging (MRI) have made it possible to accurately study cardiac morphology and function, as well as myocardial perfusion in healthy volunteers and patients with ischemic heart disease (38,39,40). T2-weighted fast spin-echo imaging, combined with perfusion-sensitive spin labeling, has also been used to measure myocardial perfusion and oxygen concentration in isolated blood-perfused rabbit hearts (41). However, MRI does not allow quantification of myocardial oxygen consumption.
PET
Regional myocardial metabolism, blood flow, and oxygen consumption can be readily studied with PET using radiolabeled metabolite analogues. The advantages of PET over other radionuclide methods are the unique ability to quantitatively measure tracer concentrations in selected tissue volumes with better spatial and temporal resolution and better sensitivity due to multiple detectors.
Measurement of Glucose and Fatty Acid Metabolism with PET
PET can be used to measure the initial steps of glucose metabolism (fluorine-18 [18F]-fluorodeoxyglucose [FDG]) (42) and the rate of fatty acid metabolism ([11C]-palmitate, [18F]-FTHA [6-triaheptecanoic acid]) (43,44). The clearance of [11C]-palmitate represents β-oxidation and oxidation in the TCA cycle. Because the rapid washout of [11C]-palmitate from myocardium partly represents the TCA cycle activity, it correlates to some extent with myocardial oxygen consumption. However, the method is sensitive to levels of tissue oxygenation and arterial fatty acids, as well as to the pattern of substrate use (45,46,47,48). Fluorine-18-FTHA is a false long-chain fatty acid substrate and inhibitor of fatty acid metabolism. After transport into the mitochondria, it undergoes initial steps of β-oxidation and is thereafter trapped in the cell. The rate of radioactivity accumulation in the myocardium would, therefore, directly reflect the β-oxidation rate of long-chain fatty acids. The uptake rate constant of [18F]-FTHA correlates well with the rate pressure product in healthy volunteers (49).
It has to be kept in mind that the rate of metabolism of one single energy substrate may reflect only a portion of the overall oxidative metabolism of the heart and depends on prevailing metabolic circumstances. Mitochondrial oxidation of intermediary substrates can also be impaired in specific conditions such as myocardial ischemia (50). The overall oxidative metabolism can, however, be quantitatively assessed with [11C]-acetate and oxygen-15 (labeled) oxygen (15O2) PET.
Assessment of Myocardial Oxygen Consumption with PET
Carbon-11-Acetate PET
FFAs and carbohydrates share the TCA cycle for oxidative metabolism (Fig. 11.3.1). The turnover rate of the TCA cycle reflects the rate of overall oxidative metabolism, and, therefore, it is an ideal site for assessing myocardial oxidative metabolism. Carbon-11-acetate is readily metabolized to carbon dioxide almost exclusively by oxidative metabolism through the TCA cycle.
First-pass extraction of acetate into the myocardium is inversely related to the myocardial blood flow (MBF) (51). In a steady state, the reported myocardial extraction fraction (EF) of radiolabeled acetate in animal models is 60% to 70% for healthy myocardium and up to 95% for ischemic myocardium (52,53). In humans, the EF of acetate is approximately 30% to 40% (54). Acetate is converted to acetyl-CoA in mitochondria, which then enters the TCA cycle. In the TCA cycle, the radiolabel in the carboxyl (C-1) position of acetate undergoes two cycle turns before the label is released as carbon dioxide (or bicarbonate in tissue) (55). Nearly all (80% to 90%) of the acetate extracted by myocardium is oxidized. The major alternative route is transamination of acetate to glutamate and aspartate via the TCA cycle intermediates (56). Therefore, the elimination rate of radiolabeled acetate reflects the overall TCA cycle flux and consequently overall oxygen consumption over a wide range of cardiac workloads (52,56).
Preparation of the Tracer
The production of [11C]-acetate is based on a method developed by Pike et al. (57). In short, [11C]-carbon dioxide is produced by the [14N]-(p,α)[11C] reaction in a medical cyclotron. Methyl magnesium bromide in diethyl ether is carbonated under nitrogen with [11C]-labeled carbon dioxide. After this, hydrochloric acid is added to the reaction during vigorous stirring, and then the phases are allowed to separate. Sodium bicarbonate (10 mL) is added to the solution, and the solution is heated under a stream of nitrogen and finally sterilized by filtration.
Imaging Protocols
The imaging protocol for [11C]-acetate is straightforward. A transmission scan is performed to correct the data for tissue photon attenuation. Carbon-11-acetate is administered as an intravenous bolus over 30 to 60 seconds (30,34). The target dose varies according to camera properties, and the dose is usually 10 to 20 mCi. The length of dynamic scanning in clinical studies varies between 20 and 49 minutes (31,58). To detect the early part of the clearance curve, the early time frames are short and the frames get longer toward the end of the study; for example, 10 × 10 seconds, 1 × 60 seconds, 5 × 100 seconds, 5 × 120 seconds, and 7 × 240 seconds (30).
Analysis of the Data
Regional Analysis
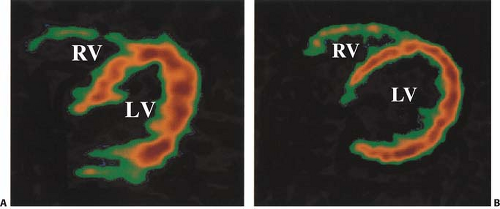
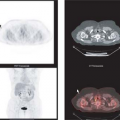
In clinical practice, several different approaches have been used to assess regional myocardial oxygen consumption. Regions of interest (ROIs) can be placed on transaxial slices (Fig. 11.3.2) or short-axis slices (59) of the LV and in some cases on the RV simultaneously (31,60,61).
Regional analysis of the data is of special interest in coronary artery disease (CAD), particularly after a myocardial infarction (MI) when there are likely to be regional differences in myocardial function and metabolism (34). Regional differences can also exist in nonischemic cardiomyopathy (62). An important clinical application of [11C]-acetate PET has been in assessing myocardial viability (58). To facilitate the comparison between PET and echocardiographic studies, one must use the same segmental division in both modalities (30,31,62,63,64,65).
Modeling of the Data
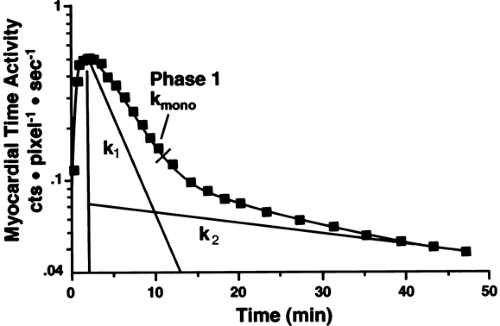
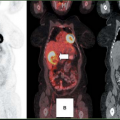
In most clinical studies, [11C]-acetate data have been analyzed by monoexponential fitting of the clearance portion of the time-activity curve (TAC) (Fig. 11.3.3) (30,31,33,34,61,66,67,68,69).
Biexponential fitting of the data (Fig. 11.3.3) has been used less frequently because the second part of the curve might be less reliable in clinical studies (70). The conventional exponential fitting
analysis does not account for the distribution of arterial input function, the recirculating [11C]-acetate, the spill-over, or the presence of metabolites. Therefore, more sophisticated compartmental and kinetic models have been introduced for estimation of myocardial oxygen consumption with [11C]-acetate PET.
analysis does not account for the distribution of arterial input function, the recirculating [11C]-acetate, the spill-over, or the presence of metabolites. Therefore, more sophisticated compartmental and kinetic models have been introduced for estimation of myocardial oxygen consumption with [11C]-acetate PET.
Because only a fraction of injected [11C]-acetate is delivered to the heart during the first pass, a recirculating amount of the tracer can be considerable and may therefore affect the shape of tissue TACs. Using computer simulations, it has been shown that the shape of the arterial input function can significantly alter the monoexponential fitting of the myocardial TAC. Buck et al. (71) introduced a two-compartment model for [11C]-acetate kinetics that accounts for tracer recirculation. The model requires assessment of an input function and correction for tracer metabolites and spill over effects. The model-derived parameter k2 correlated closely with directly measured MMRO2. This method has been successfully used in patients with congestive heart failure (CHF) (34) and patients with acute MI (72). Raylman et al. (73) were able to improve the accuracy of the fitted k2 parameter by simultaneously fitting data from multiple ROIs. Sun et al. (74) tested a comprehensive tracer kinetic model (six-compartment model) for [11C]-acetate kinetics in dogs, which takes into account differences in input function. This model yielded accurate MMRO2 measurements compared with invasive measurements. However, this model has not been validated in humans. The same is true for the compartmental model introduced by Ng et al. (75).
Although these models are theoretically more accurate than the traditional fitting methods, their complexity and the need for
additional assessments, such as arterial blood sampling, limit their feasibility in clinical studies.
additional assessments, such as arterial blood sampling, limit their feasibility in clinical studies.
Experimental Studies and Validation of the Method
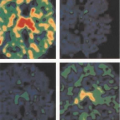
The detected TAC reflecting the clearance of [11C]-acetate from the myocardium can be either monoexponential or biexponential depending on the level of oxidative metabolism. The curve is usually monoexponential at rest, during hypoxia and ischemia, and it is usually biexponential during increased metabolic demands such as during dobutamine infusion (53,70). The decay constant of the initial component of the clearance curve has been shown to be linearly related to myocardial oxygen consumption. The accuracy of [11C]-acetate PET in measuring myocardial oxygen consumption in the LV has been validated in experimental studies in isolated perfused rabbit hearts, rat hearts, and dogs (52,53,56,76) (Fig. 11.3.4). In humans, the elimination rate of [11C]-acetate has been shown to correlate with indirect estimates of myocardial oxygen consumption both in the LV (61,69) and in the RV (60). The elimination rate of [11C]-acetate also correlates well with directly (invasively) measured myocardial oxygen consumption in healthy volunteers (77,78) and patients with dilated cardiomyopathy (34,79). Although variations in the pattern of substrate utilization can alter the ratio of TCA cycle flux to oxygen consumption, the magnitude of the change is insignificant (76).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree