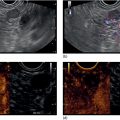
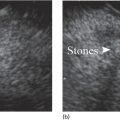
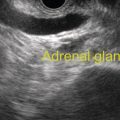
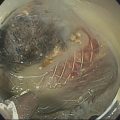
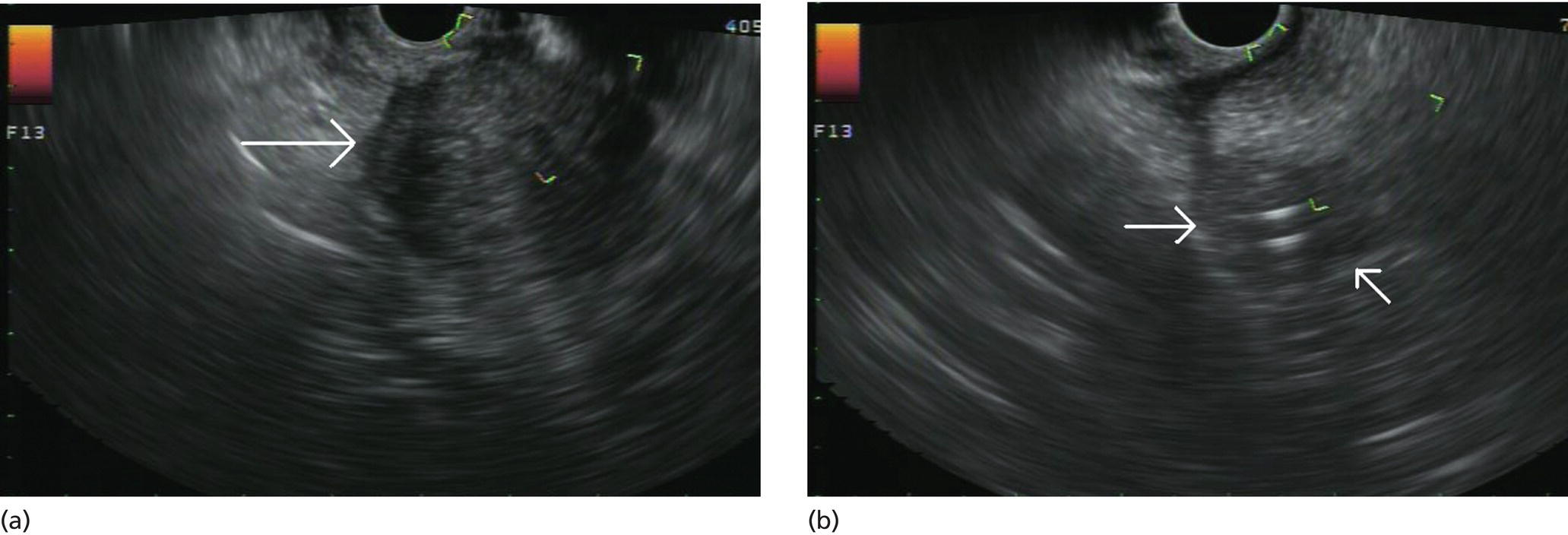
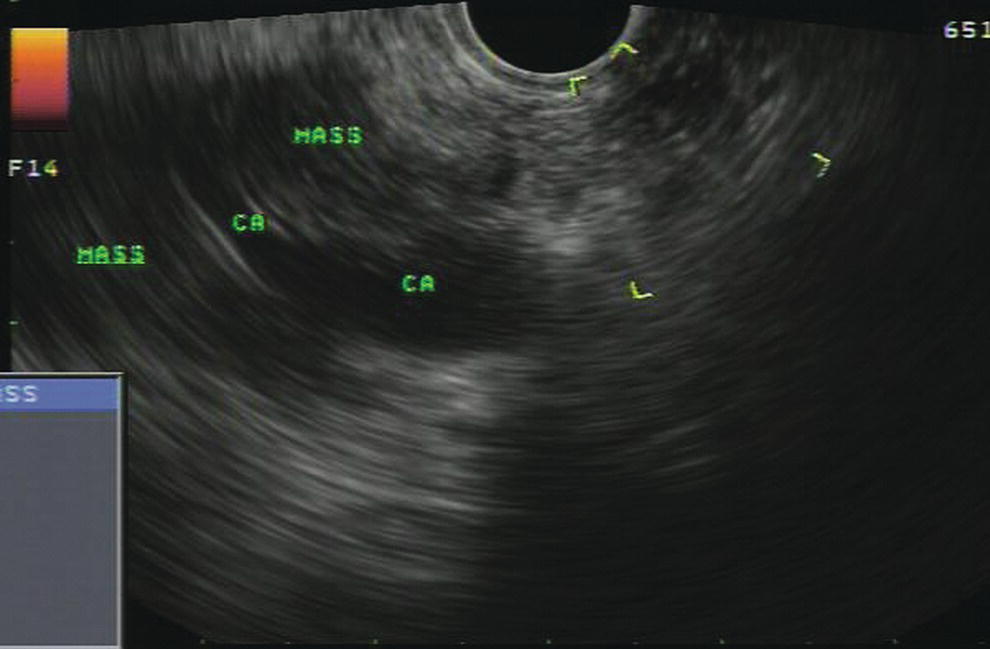
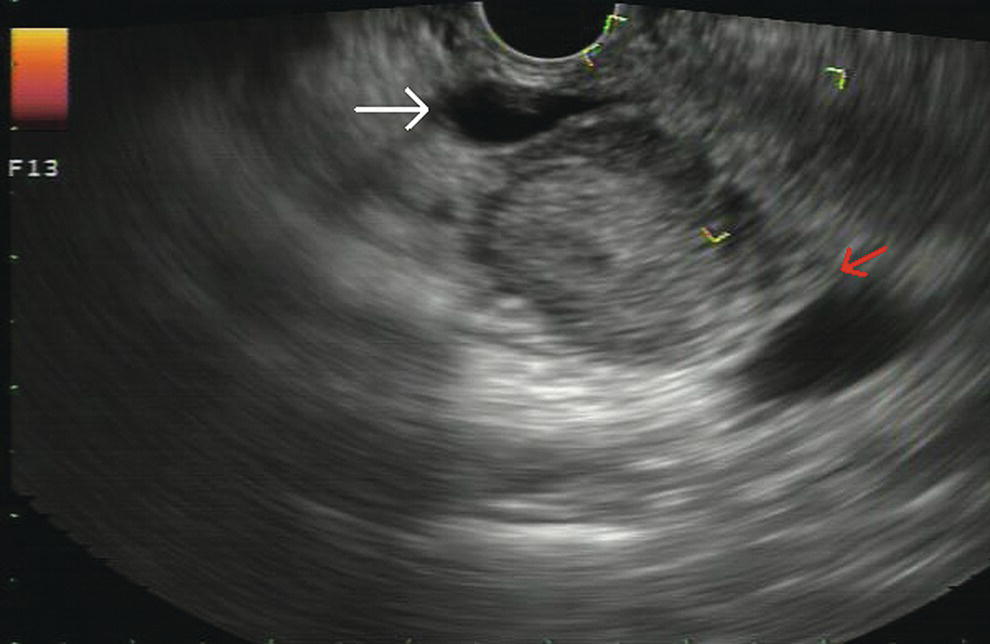
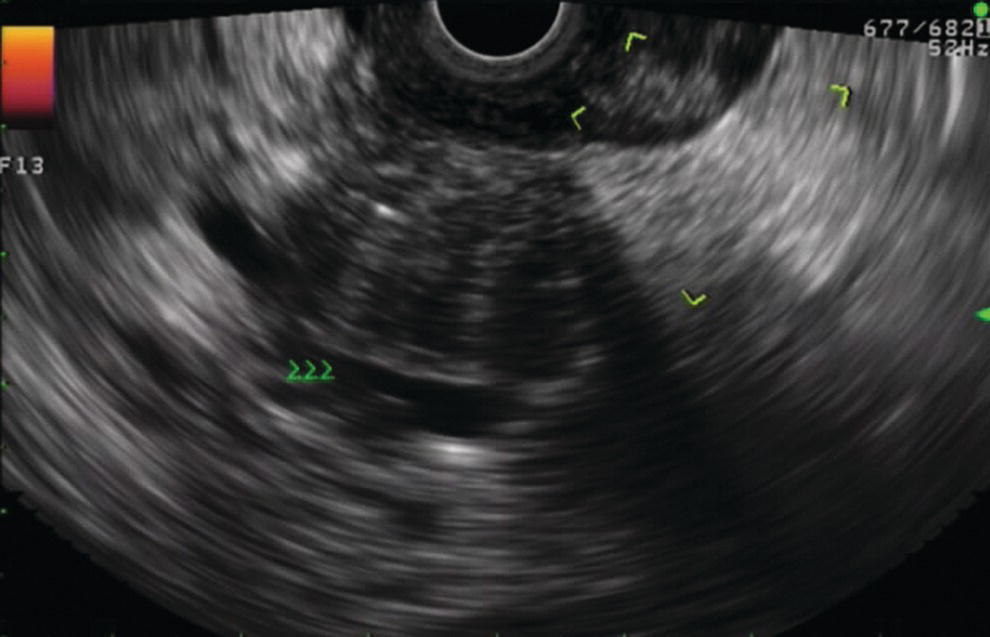
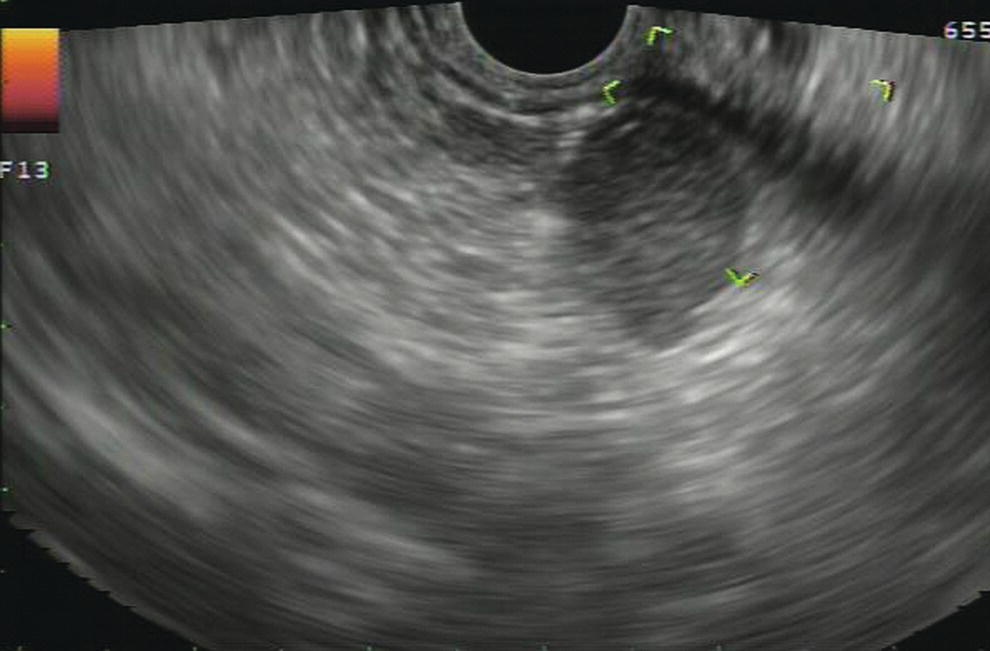
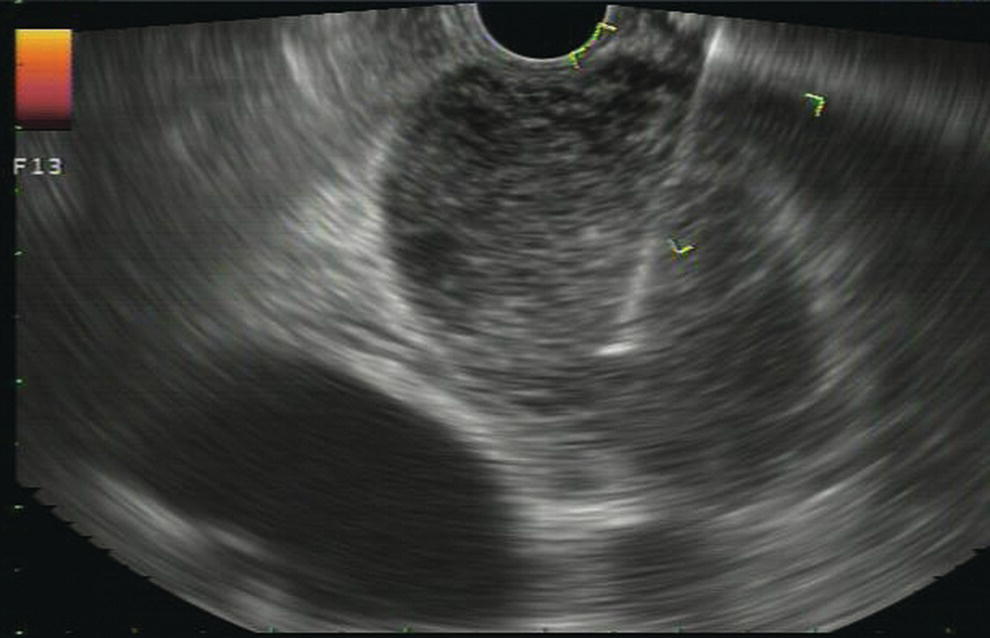
Douglas G. Adler Peak Gastroenterology Associates, Colorado Springs, CO, USA, Adenocarcinoma accounts for 90% of all pancreatic tumors. The highest cure rate is seen in patients with lesions that are localized to the pancreas and do not involve significant local vascular structures. Unfortunately, only 15–20% of patients have potentially resectable disease at the time of diagnosis [1]. Surgery has been and remains the most effective treatment for pancreatic cancer, although 5‐year survival rates remain low at 18–33% even in patients who progress to surgery, most commonly following neoadjuvant therapy with chemotherapy, radiation therapy, or a combination thereof [2,3]. Accurate staging of patients with pancreatic adenocarcinoma is critical and allows patients to be appropriately selected to undergo all potential treatments including surgery, chemotherapy, radiation therapy, or palliative care. Endoscopic ultrasound (EUS) is a well‐established and minimally invasive technique that has proved invaluable for both accurate locoregional staging of pancreatic adenocarcinoma and as a means of providing tissue from tumors and lymph nodes, thus allowing for a definitive diagnosis. The overall staging accuracy of EUS in patients with pancreatic adenocarcinoma is approximately 90–100%. In general, EUS is superior to computed tomography (CT) for the detection of pancreatic cancers. For small pancreatic masses (defined as lesions ≤16 mm), the sensitivity of EUS for tumor detection approaches 100% as compared to 66% using multidetector CT. As determined by EUS, accuracy for resectability is 88–100%. If CT or magnetic resonance imaging (MRI) reveals metastatic disease, then staging is complete (the patient has stage IV disease), and EUS may not be required, at least for diagnostic purposes. In patients with metastatic disease, EUS with fine needle aspiration (FNA) can still be performed if a definitive tissue diagnosis is desired. In patients with metastatic disease, EUS may also be used to perform celiac neurolysis. The sensitivity, specificity, positive predictive value, and negative predictive value of EUS‐guided pancreatic FNA for the diagnosis of malignancy in patients with pancreatic cancer has been reported to be 64%, 100%, 100%, and 16%, respectively [4] (Figures 22.1 and 22.2). Recent work on the EUS evaluation of extravascular migratory metastases has highlighted the fact that tumor cells can migrate along vessels and these can be identified and sampled via EUS. Work in this arena is ongoing [5]. Elastography, the use of sound waves to assess the stiffness of tissue, has been used to guide EUS‐FNA or EUS core biopsies to target areas of viable tumor and to minimize sampling of necrotic tissue [6]. Elastography is widely used in Europe, less so in the USA. It should be noted that EUS‐guided fine needle biopsy (EUS‐FNB) is now widely performed. This allows core samples to be obtained for histologic evaluation (and not just cytologic evaluation). Tissue can also be used for personalized medicine by oncologists [7,8]. As mentioned, detection of vascular involvement in patients with pancreatic adenocarcinomas is critical for staging. Unfortunately, the definition of “vascular involvement” invasion has not been uniformly defined in the literature or in clinical practice, and different physicians, institutions, and research studies define this term differently. When evaluating peripancreatic vessels via EUS, tumor can be seen to contact vessels with or without the loss of a clear tissue plane (a.k.a. echoplane), be adjacent to vessels, invade blood vessels with tumor visualized in the vascular lumen, thrombose vessels, and/or to encase blood vessels. All of these are considered vascular involvement, but individual surgeons and oncologists may differ about which of these findings may exclude patients for surgery. If there is vascular involvement/invasion between the tumor and the celiac artery and/or the superior mesenteric artery, the patient has unresectable disease (Figure 22.3). Vascular invasion of the splenic artery or splenic vein are typically not contraindications to surgical intervention. With respect to the portal vein and/or the superior mesenteric vein, vascular invasion into the portal vein, the superior mesenteric vein, or the confluence of these vessels is not a contraindication to surgery, but advanced surgical techniques such as vascular grafting and/or reconstruction may be required for successful pancreaticoduodenectomy (Figures 22.4 and 22.5; Video 22.1). In patients with pancreatic adenocarcinoma, vascular staging accuracy for EUS has been reported to be 100% versus 81% for CT in one study. Another similar study found EUS to have a sensitivity, specificity, positive predictive value, and negative predictive value for determining superior mesenteric vein and portal venous involvement in patients with pancreatic adenocarcinoma to be 81%, 86%, 87%, and 80%, respectively. This same study also demonstrated that for superior mesenteric artery tumor involvement the sensitivity and specificity, positive predictive value, and negative predictive values have been reported to be 17%, 67%, 17%, and 67%, respectively. These lower numbers reflect that the superior mesenteric artery can be difficult to fully track via EUS once it diverges significantly from the aorta. The limited ability to see the superior mesenteric artery well on EUS is perhaps the greatest limitation of the technique. A meta‐analysis found the overall sensitivity of EUS for diagnosing vascular invasion overall to be 73% with a specificity of 90% [9]. Figure 22.1 (a) Representative EUS image of a large pancreatic adenocarcinoma (arrow). The tumor is a well‐demarcated, hypoechoic mass lesion in the body of the pancreas. (b) Representative EUS image of a small pancreatic adenocarcinoma (arrows). The patient has a 1.5‐cm lesion in the head of the pancreas surrounding the common bile duct, which is seen to contain a plastic stent. The walls of the plastic stent appear as bright hyperechogenic lines. Figure 22.2 EUS‐guided FNA of a pancreatic adenocarcinoma with a 22‐gauge needle. Figure 22.3 EUS image of a pancreatic adenocarcinoma in the body of the pancreas encasing the celiac artery (CA). Figure 22.4 A 2‐cm pancreatic head mass seen to abut and compress the common bile duct (white arrow, top of image) and to abut the portal vein (bottom of image) with an intact tissue plane between the mass and the vessel (red arrow). Figure 22.5 Large pancreatic cancer in the head of the pancreas seen to encase the superior mesenteric vein (green arrowheads). Source: Aloka. The presence or absence of malignant peripancreatic lymphadenopathy does not play a definitive role in determining if a pancreatic cancer is resectable or unresectable, but the presence of malignant adenopathy can have a powerful effect on overall management. If a cancer appears to be potentially resectable, the identification of malignant lymph nodes may be a justification for neoadjuvant therapy. When imaged via EUS, malignant lymphadenopathy is suggested by the presence of lymph nodes that are greater than 1 cm in size, hypoechoic, have distinct margins, and are round in shape. Individually, none of these characteristics determine the presence of malignant cells, but when all four of these features are present in the same lymph node, the accuracy for predicting malignant involvement is 80% [10]. Sensitivity, specificity, positive predictive value, and negative predictive value for the detection of celiac axis, portal vein, hepatic artery and superior mesenteric artery lymph nodes via EUS are 44%, 93%, 80%, and 72%, respectively (Figures 22.6 and 22.7). Figure 22.6 A 15‐mm peripancreatic lymph node. Node is hypoechoic, round, well demarcated, and greater than 1 cm in size, all of which suggests malignancy. Figure 22.7 EUS‐guided FNA of a large, 4‐cm peripancreatic node in a patient with pancreatic adenocarcinoma. Cytology revealed malignant cells. EUS evaluation of peripancreatic lymphadenopathy is superior to that of CT [11,12]. While not all malignant appearing nodes are sampled, if the appearance of a lymph node is indeterminate for malignant involvement, EUS‐guided FNA or FNB of the node in question can be performed for a definitive nodal evaluation. The overall sensitivity, specificity, and accuracy of detection of malignant lymph nodes by EUS are approximately 85%, 100%, and 89%, respectively [13]. Despite its value in patients with pancreatic adenocarcinoma, EUS has some significant limitations. Pancreatic adenocarcinomas may be difficult to detect via EUS (and, of note, CT or MRI) in patients with chronic pancreatitis (due to baseline tissue distortion) or if the tumor is isoechoic with the surrounding pancreatic parenchyma [14,15]. As already mentioned, EUS is relatively insensitive for the detection of superior mesenteric artery involvement. Complete visualization of the entire pancreas can sometimes be difficult, especially in patients with prior bariatric surgery or partial gastrectomy. The pancreatic genu and uncinate process can be challenging to completely image, especially in patients with large body habitus. Potential complications from EUS with or without FNA can include acute pancreatitis (0–2%), perforation (0.03%), infection/febrile episode (1%), and significant hemorrhage (1.3–4%) [16,17]. The number of EUS practitioners, while steadily increasing, still remains concentrated in large medical centers. One study on EUS training volumes revealed that most three‐year gastrointestinal fellows and many fourth‐year advanced endoscopy fellows are currently receiving insufficient training in EUS. Further limiting the spread of EUS to the general community, training in EUS is not offered at 14% of US gastrointestinal fellowship programs [18]. In general, the best results are obtained from the most experienced operators. Pancreatic adenocarcinoma remains a commonly encountered clinical entity. Accurate staging and tissue diagnosis are critical for formulation of optimal management plans. EUS offers high accuracy for staging of pancreatic adenocarcinoma, provides excellent tissue acquisition, and is essentially unrivaled for the detection of small tumors. EUS is also highly effective in evaluating patients for the presence of malignant lymphadenopathy and in assessing vascular involvement.
22
Pancreatic Adenocarcinoma
Introduction
Tumor identification and diagnosis via fine needle aspiration or fine needle biopsy
Evaluation of vascular invasion
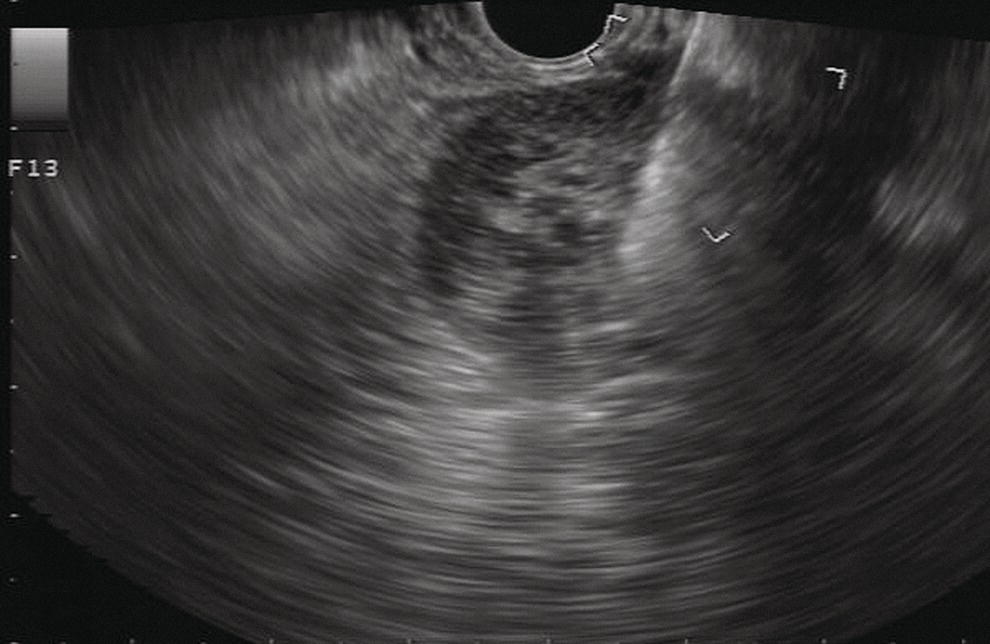
Evaluation of peripancreatic lymphadenopathy
Limitations and complications of EUS in patients with pancreatic cancer
Conclusion
References
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree