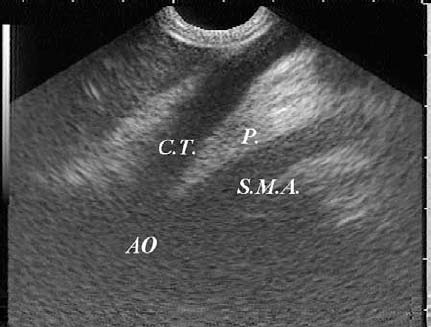
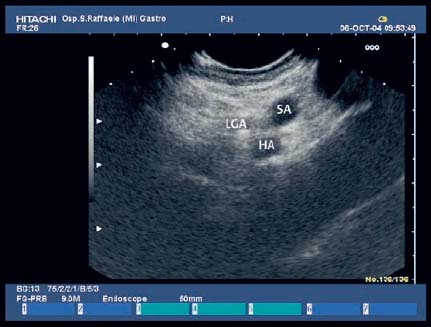
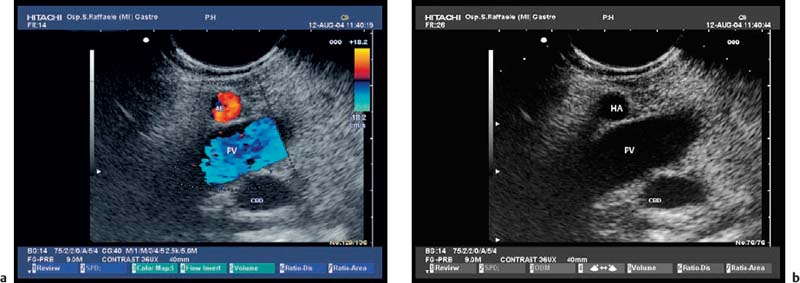
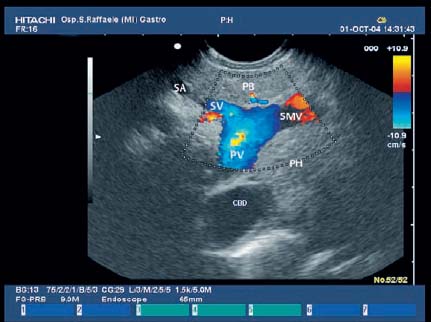
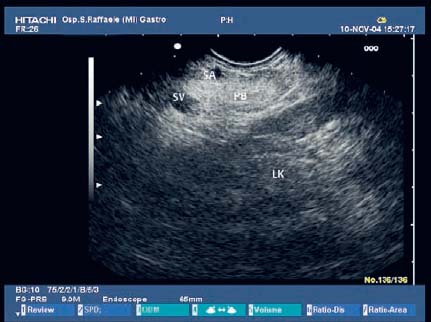
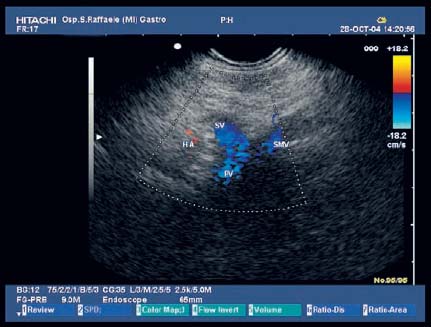
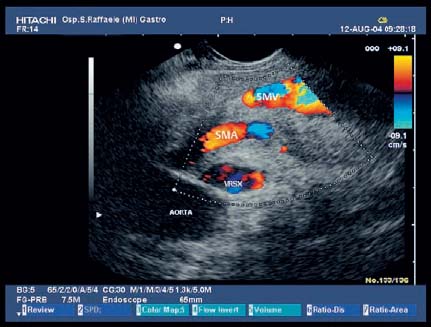
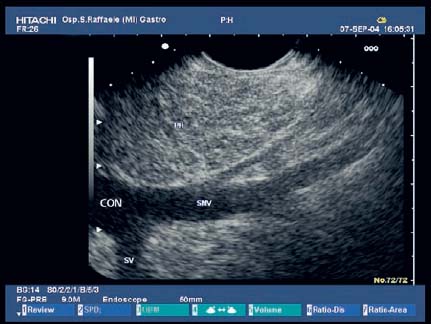
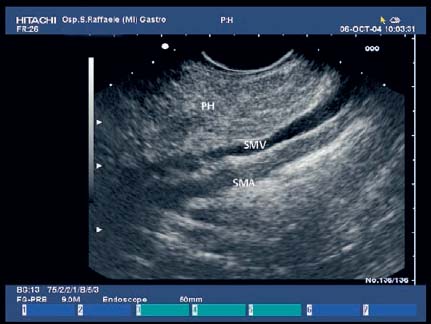
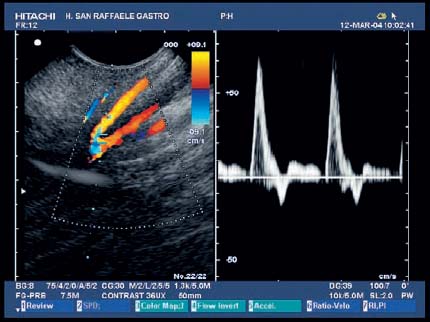
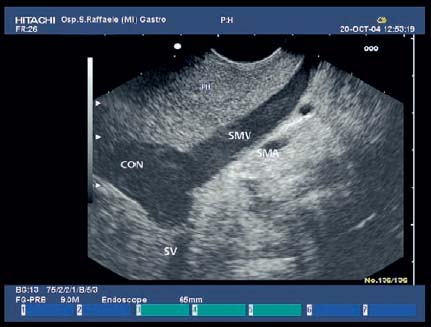
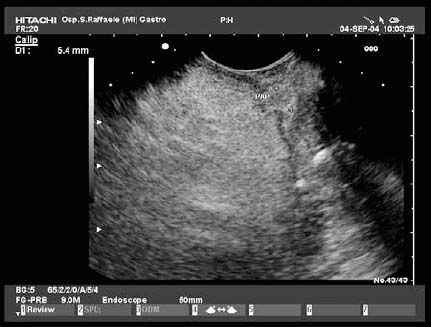
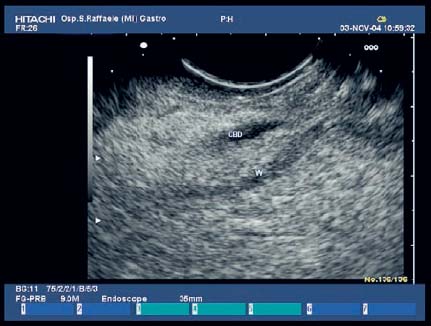
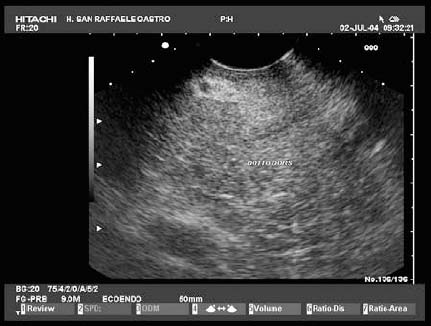
17 Pancreatic Adenocarcinoma: the Role of Endoscopic Ultrasonography Pancreatic cancer is the fourth leading cause of cancer death in Western countries. It is unusual in persons under the age of 45, while after the age of 50 its frequency increases linearly. The increase in the incidence rate ranges from two per 100 000/year in patients aged 40–44, to 67 per 100 000/year in patients older than 75.1 At the age of 70, the mortality due to pancreatic cancer is ≈ 60 deaths per 100 000 persons per year. Some 74000 new cases of pancreatic cancers were diagnosed in 2000 in Europe.2 Screening in high-risk populations. Effective early detection and screening for average-risk populations are currently not available, but efforts are focusing on early screening of selected high-risk patients, who account for ≈ 10% of patients with pancreatic cancer. These high-risk groups include patients with chronic pancreatitis, kindreds with two or more first-degree relatives affected by pancreatic cancer, and patients with hereditary pancreatitis, Peutz-Jeghers syndrome, cystic fibrosis, familial breast cancer syndrome, and familial atypical multiple mole melanoma. At present, a multimodal screening approach involving endoscopic ultrasound, computed tomography, and/or magnetic resonance appears to be the most effective method of screening for pancreatic cancer in high-risk patients.3,4 Rulyak et al. investigated the cost-effectiveness of pancreatic cancer screening in familial pancreatic cancer patients. The authors compared one-time screening with endoscopic ultrasonography (EUS) for pancreatic dysplasia with no screening, in a hypothetical cohort of 100 members of families with pancreatic cancer. EUS was defined as abnormal if one or two of the following abnormalities were present: heterogeneous parenchyma with echogenic foci, hypoechoic nodules, hyperechoic main duct wall, or discrete masses. Patients with abnormal EUS underwent endoscopic retrograde cholangiopancreatography (ERCP), and if ERCP confirmed the abnormality, they underwent total pancreatectomy. This endoscopic screening approach was cost-effective, with an incremental cost-effectiveness ratio of $ 16 885 per life-year saved. Screening was more cost-effective as the probability of dysplasia and the sensitivity of EUS and ERCP increased. Screening was cost-effective if the prevalence of dysplasia was greater than 16% or the sensitivity of EUS was greater than 84%. In conclusion, endoscopic screening for carefully selected members of families with pancreatic cancer appears to increase patients’ life expectancy in a cost-effective manner.5 Etiological and clinical information. Pancreatic cancers can arise from both the exocrine and endocrine cells. Among pancreatic tumors, ≈ 95% develop from the exocrine portion of the pancreas, including the ductal epithelium, acinar cells, connective tissue, and lymphatic tissue. Approximately 75% of pancreatic carcinomas occur in the head or neck of the pancreas, 15–20% occur in the body of the pancreas, and 5–10% occur in the tail. Typically, pancreatic cancer first metastasizes to regional lymph nodes, and then to the liver and less commonly to the lungs. It can also directly invade surrounding visceral organs such as the duodenum, stomach, and colon. It spreads rapidly, is seldom detected in the early stages, and is extremely difficult to treat. Symptoms such as jaundice, abdominal pain, and weight loss may not appear until the disease is quite advanced. By that time, the cancer is likely to have spread to other tissues and surgical removal is no longer possible. This is why the conditions has been called the “silent killer.” The annual mortality rate approximates the annual incidence rate, reflecting the short survival time associated with pancreatic cancer.6 Overall, fewer than 5% of patients are still alive 5 years after the diagnosis. The chances of achieving a 5-year survival are best with early diagnosis and treatment, although the odds are still slim. The EUROCARE study analyzed the survival in 49 074 patients with pancreatic cancer at 1, 3, and 5years from the diagnosis: the figures were 15.9%, 5.4%, and 4.1%, respectively.7 The data were similar for men and women. The median survival time for patients with advanced cancer is ≈ 4–6 months. For many years, little was known about the pancreas; it was one of the most difficult organs to study, due to its deep location in the abdomen, often inaccessible to traditional ultrasound, and too distant to biopsy. The complexity of pancreatic disease and the richness of the organ’s anatomical relationships made the approach to this gland a challenge even for the best surgeons. Researchers are now beginning to understand the genetic basis and the pathogenesis of the disease, and imaging technologies are developing new and sophisticated methods of diagnosing and staging pancreatic cancer. Ultrasound, computed tomography (CT), and ERCP are the conventional radiologic modalities in the management of pancreatic cancer. Endoscopic ultrasound was developed in the 1980s after high-frequency ultrasound transducers were combined with endoscopes to overcome the physical limitations of transabdominal ultrasonography (which is obstructed by fat, air, and bone). Initially, EUS was used to confirm and stage solid masses in which a suspicion of malignancy had been raised by other imaging techniques or in the clinical examination. As technology improved, dedicated accessories were developed, and endosonographers’ experience grew, the application of EUS continued to expand. Its widespread use has revolutionized the management of pancreatic disease. It is now also used to diagnose and follow up benign or borderline diseases, and it is often required for evaluation of focal enlargement of the gland, in the absence of a definite mass with other imaging methods. It has been demonstrated that a CT finding of fullness or enlargement of the pancreas may be a sign of malignancy and that EUS may then be able to identify a mass.8,9 In this case, fine-needle aspiration biopsy (FNA) is carried out with endosonographic guidance. A recent article has elucidated the potential benefits and drawbacks of EUS in the diagnosis, staging, and assessment of resectability of pancreatic cancer, from the origins of the technique up to the present day.10 The probe, which can be positioned in the esophagus, stomach, and duodenum, provides ultrasound images of the gastrointestinal wall, distinguishing the different parietal layers, and producing transmural images of the surrounding structures. Two types of echoendoscope were initially developed: the radial-scanning instrument, with scans perpendicular to the scope’s axis, providing radial 360° images similar to those of CT; and the convex linear-array instrument, with a scanning plane parallel to the longitudinal axis of the echoendoscope. The radial echoendoscope was first developed by the Olympus Corporation, while Pentax and Hitachi developed the convex linear-array probe, with an oblique forward optical view. No differences between the two types of instrument have been found with regard to the accuracy of pancreatic cancer staging.11 Fig. 17.1 The origin of the celiactrunk (CT) and superior mesenteric artery (SMA) from the aorta (AO); imaging from the proximal stomach. P, pancreas. The linear-array echoendoscope is described in this chapter, as it is the instrument used for interventional procedures. It has a biopsy channel, the size of which varies from model to model. The probe is sheathed with a latex balloon that can be filled with water to allow acoustic coupling. The tip of the instrument is equipped with an elevator to angle the accessories exiting from the biopsy channel. The transducer operates at different frequencies, from 5 to 10 MHz. The frequency is inversely proportional to the penetration depth of ultrasound; at 5 MHz, the ultrasound penetrates ≈ 8 cm, while at 10 MHz the penetration field of ultrasound is ≈ 4 cm. The Hitachi console provides color and power Doppler images. The patient is positioned in the left lateral decubitus position, as for any standard upper endoscopy procedure. Informed consent is obtained. Conscious sedation can be administered with midazolam and meperidine. The ideal condition is deep sedation with assistance from an anesthesiologist. The patient is monitored during and after the procedure. Examination of the pancreas starts from the proximal stomach. The most important landmark here is the descending aorta, with the emergence of the celiac trunk and, a few centimeters below, the superior mesenteric artery (Fig. 17.1). The celiac trunk divides into three branches—the left gastric artery, the splenic artery, and the hepatic artery (Fig. 17.2). Just past the cardia, the tip of the echoendoscope is placed against the lesser curvature. From this point, a scan from the hepatic hilum (Fig. 17.3) with the portal vein as far as the splenomesenteric confluence (Fig. 17.4) is obtained by rotating the wrist clockwise on the instrument. The splenic vein and the splenic artery are important landmarks for imaging the body of the pancreas, where the main pancreatic duct is seen as two parallel hyperechoic lines; its lumen is usually virtual, but it may vary from 1 to 3 mm. (Figs. 17.5 and 17.6). Fig. 17.2 The celiac trunk division with its branches, the left gastric artery (LGA), splenic artery (SA), and hepatic artery (HA). Fig. 17.3a, b a The hepatic hilum from the stomach. CBD, common bile duct; HA, hepatic artery; PV, portal vein. Color flow image. b Without color flow. Fig. 17.4 The splenic vein (SV) and superior mesenteric vein (SMV) entering the confluence. CBD, common bile duct; PB, pancreatic body; PH, pancreatic head; PV, portal vein; SA, splenic artery. The splenic artery has a cephalic location, and its course is more serpiginous than that of the vein. The splenic vein is larger and oval in shape, while the artery is round. As the splenic vessels are followed along the posterior wall of the stomach to the splenic hilum, and as the tip is rotated more to the right, the tail of the pancreas is visualized as far as the spleen and, below, the left kidney. For the tail to be seen, the instrument has to be withdrawn slightly, as the tail courses upward and leftward. The pancreatic neck is imaged from the stomach as the tip is advanced further into the antrum. The main landmark, the superior mesenteric artery, imaged longitudinally, courses posterior to the pancreas, parallel to the splenomesenteric confluence. From this position, the probe displays the pancreatic neck and visualizes one of the most important crossroads for the staging of vascular involvement in patients with pancreatic cancer: the portal vein, the splenomesenteric confluence, with the splenic vein and the superior mesenteric vein, and the superior mesenteric artery (Fig. 17.7; see also Fig. 17.4). Fig. 17.5 View of the pancreatic body (PB) from the stomach. The splenicvessels are important landmarks for visualizing the body and the tail of the pancreas. The splenic artery is proximal to the splenic vein. LK, left kidney; SA, splenic artery; SV, splenic vein. If there is portal vein obstruction, collaterals may be seen along the gastric and duodenal wall; color Doppler is helpful for recognizing these vessels. The transducer is then advanced to the descending duodenum, past the ampulla of Vater, and the instrument is straightened into the short position. Air in the duodenal lumen is suctioned, and the latex balloon on the probe is inflated with water; the duodenal lumen may also be filled with water to improve acoustic coupling. From this position, a full scan of the pancreatic head and uncinate process is obtained (Fig. 17.8)—from the mesenteric vessels (vein and artery), with the echoendoscope rotated to the right, to the aorta, with rotation to the left (Figs. 17.9, 17.10, and 17.11). Fig. 17.6 The pancreatic body, with the splenic vein (SV) and the superior mesenteric vein (SMV) forming the portal vein (PV). HA, hepatic artery. Fig. 17.7 A color flow image of the abdominal vessels from the posterior wall of the stomach. The pancreatic body is seen with the superior mesenteric artery (SMA) emerging from the aorta and the superior mesenteric vein (SMV), and the left renal vein (VRSX) crossing the aorta below the SMA. Fig. 17.8 The uncinate process seen from the duodenum. The portal confluence (CON) is seen with its vessels—the superior mesenteric vein (SMV) and splenic vein (SV). PH, pancreatic head. Fig. 17.9 The pancreatic head (PH) from the duodenum, with the mesenteric vessels, the superior mesenteric vein (SMV) and the superior mesenteric artery (SMA). Fig. 17.10 A color and fast Fourier transform (FFT) Doppler image of the mesenteric vessels seen from the duodenum. The probe is maneuvered and withdrawn with fine movements. The superior mesenteric vein is followed as far as the confluence. Here, the neck of the pancreas is also visualized from the duodenum, and this image complements the previous scan obtained from the stomach. Two different areas of the pancreatic head can be identified, due to their different embryogenesis—the dorsal and the ventral pancreas. Their echogenicity differs; the ventral part may appear more hypoechoic, mimicking a pseudotumor, although its structure is homogeneous, while the dorsal part is more hyperechoic, as it is richer in interacinar fat tissue. The ampulla of Vater is seen as a hypoechoic, well-delineated structure (Fig. 17.12), from which the two ducts—the common bile duct (upper) and the main pancreatic duct (lower)—can be traced and followed into the pancreatic head (Fig. 17.13). While the ampulla is being imaged, one must ensure that the probe is not pressed too hard against the duodenal wall, to avoid compression artifacts. The common bile duct can be followed as far as the hepatic hilum, with the tip being withdrawn and lifted. As the tip is withdrawn from the major ampulla, the minor ampulla and Santorini duct (the accessory pancreatic duct) are imaged (Fig. 17.14). Fig. 17.11 The uncinate process with the veins and, more deeply, the superior mesenteric artery (SMA), which is located below the portal confluence (CON). PH, pancreatic head; SMV, superior mesenteric vein; SV, splenic vein. Fig. 17.12 Scanning of the normal papilla of Vater (PAP) from the duodenum. Fig. 17.13 View of the common bile duct (CBD) and distal pancreatic duct (Wirsung duct, W) in the pancreatic head from the descending duodenum. The two ducts are shown in a longitudinal section that passes into the papilla, the smooth, hypoechoic area on the right of the image. The normal pancreatic parenchyma has a characteristic homogeneous “salt-and-pepper” echo texture. Its echo structure is similar to that of the liver in young people. In older people and in the obese, it is more hyperechoic. Fig. 17.14 A view of the Santorini duct (SD, the accessory pancreatic duct) from the descending duodenum. Once the papilla of Vater has been visualized, the transducer is withdrawn further, with fine movements; the minorpapilla comes into view, and the Santorini duct can be followed into the pancreatic head. The prognosis in cancer depends on how advanced the tumor is at the time of diagnosis. Accurate staging is essential to allow the best treatment decisions to be made, particularly in selecting patients for surgery and patients for chemotherapy or palliative procedures. Pancreatic adenocarcinoma is classified using the tumor, node, and metastases (TNM) staging system which was modified by the American Joint Committee on Cancer in 2002.12 This classification assigns tumors that are invading the portal venous system (previously staged as T4) to T3. Although surgery is technically feasible, invasion of the portal vein, superior mesenteric vessels, and celiac trunk usually precludes surgical resection13 (Tables 17.1 and 17.2).
Tumor (T) | |
TX | Primary tumor cannot be assessed |
T0 | No evidence of primary tumor |
Tis | Carcinoma in situ |
T1 | Tumor limited to the pancreas, 2 cm or smaller in largest diameter |
T2 | Tumor limited to the pancreas, larger than 2 cm in largest diameter |
T3 | Tumor extension beyond the pancreas (i.e., duodenum, bile duct, portal or superior mesenteric vein) but not involving the celiac axis or superior mesenteric artery |
T4 | Tumor involves the celiac axis or superior mesenteric arteries |
Regional lymph nodes (N) | |
NX | Regional lymph nodes cannot be assessed |
N0 | No regional lymph-node metastasis |
N1 | Regional lymph-node metastasis |
Distant metastasis (M) | |
MX |
|