Pancreatic and Hepatobiliary Cancers
Oleg Teytelboym
Dominique Delbeke
Richard L. Wahl
Pancreatic Carcinoma
Pancreatic ductal adenocarcinoma is the most common type of pancreatic cancer, accounting for 5% of cancer related deaths in the United States (1). Pancreatic adenocarcinoma is characterized by diagnosis occurring when the disease is quite advanced, aggressive behavior with invasion into adjacent soft tissues, lymph nodal and hepatic metastasis, and a generally poor prognosis. Early diagnosis in a localized state and prompt and complete surgical resection offer the only opportunity for cure. The preoperative diagnosis, staging, and treatment of pancreatic adenocarcinoma remain challenging. Imaging of pancreatic carcinoma can be separated into several distinct tasks: (a) detecting the presence of a pancreatic mass, (b) lesion characterization, (c) image guidance for biopsy, (d) detection of unresectable disease, (e) detection of recurrent disease, and (f) monitoring therapy response.
Limitations of Conventional Imaging Modalities
Pancreatic cancer is most commonly initially detected by computed tomography (CT), presenting as a hypoenhancing mass (on intravenous [IV] contrast CT), causing obstruction of the common bile duct and or pancreatic duct. CT currently is the most common diagnostic imaging modality used in the preoperative diagnosis and staging of pancreatic cancer. Application of dual-phase CT protocols has been reported to demonstrate 97% sensitivity and 92% positive predictive value for pancreatic tumor detection, 75% sensitivity for hepatic metastasis, 54% sensitivity for nodal metastasis, with an overall 91% accuracy for determining nonresectability (2). Evolution of CT technology has significantly improved assessment of vascular involvement and invasion of adjacent organs. Recent studies have demonstrated negative predictive value of 100% for detection of vascular invasion and 87% negative predictive value for overall resectability (3). However, since many pancreatic cancers recur, including locally, the true definition of “resectability” probably shows somewhat limited good performance.
Unfortunately, interpretation of the CT scan is sometimes difficult in the setting of mass-forming pancreatitis or questionable findings such as enlargement of the pancreatic head without definite signs of malignancy or a discrete mass (4,5). Further, a variety of characteristics have been described to determine vascular invasion, which is on a continuum of certainty. The diagnosis of locoregional lymph node metastases is also difficult with CT because such nodes often are small. In addition, subcentimeter hepatic metastases cannot reliably be differentiated from cysts and hemangiomas (6).
The diagnostic performance of magnetic resonance imaging (MRI) remains similar to that of CT (7,8,9,10). Even with the latest technology improvements, MRI demonstrates sensitivity of 86% and specificity of 89% for detection of pancreatic tumors (11). Although MRI is probably superior to CT for evaluation of hepatic metastases, lower spatial resolution makes detection of small lymph nodes even more difficult.
Endoscopic ultrasonography (EUS) has a limited field of view but offers the possibility of tissue diagnosis with fine-needle aspiration (FNA) biopsy, with reported diagnostic yield of 68% and diagnostic accuracy of 74.4% (12). However, reported overall diagnostic rate, sensitivity, and negative predictive value of FNA biopsies guided using CT (97.7%, 94.9%, and 60%, respectively) are not significantly different from those guided using EUS (88.9%, 85%, and 57.1%, respectively) (13). Although both methods of biopsy offer definitive tissue diagnosis, there remains a substantial rate of inconclusive results. Biopsy accuracy is, of course, dependent on sampling the proper tissue, and false-negative biopsies can occur due to sampling errors.
The difficulty in making a preoperative diagnosis is associated with two types of adverse outcomes. First, less-aggressive surgeons may abort attempted resection due to a lack of tissue diagnosis. This is borne out by the significant rate of “reoperative” pancreaticoduodenectomy performed at major referral centers (14,15,16). A second type of adverse outcome, generated by failure to obtain a preoperative diagnosis, occurs when more aggressive surgeons inadvertently resect benign disease. This is particularly notable in those patients who present with suspected malignancy without an associated mass on CT scan. This has been reported to occur in up to 55% of patients in some series (17). Another adverse outcome can be aggressive surgical resection for “cure” of disease that is already systemically metastatic, if diagnostic testing is inadequately sensitive.
18-F Fluorodeoxyglucose PET in the Preoperative Diagnosis of Pancreatic Carcinoma
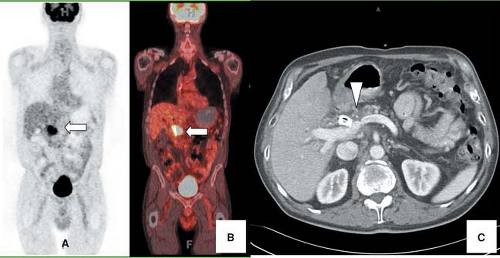
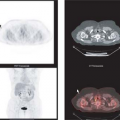
Metabolic imaging with fluorine-18 ([18F])-fluorodeoxyglucose (FDG) positron emission tomography (PET) can be used to improve the accuracy of the preoperative diagnosis of pancreatic carcinoma by differentiating benign and malignant pancreatic masses (Fig. 8.15.1). Most malignancies, including pancreatic carcinoma, demonstrate increased glucose utilization due to an increased number of glucose transporter (GLUT) proteins and increased hexokinase and phosphofructokinase activity (18,19). There is evidence that the overexpression of GLUTs by malignant pancreatic cells contributes to the increased uptake of FDG by these neoplasms (20,21).
In 10 studies (22,23,24,25,26,27,28,29,30,31), the performance of FDG PET to differentiate benign from malignant lesions ranged from 85% to 100% for sensitivity, 67% to 99% for specificity, and 85% to 93% for accuracy. In most of these studies, the accuracy of FDG PET imaging was superior to that of CT. For example, in the study of Delbeke et al. (31), the sensitivity and specificity of FDG PET imaging was 92% and 85%, respectively, compared with 65% and 62%, respectively, for CT. In another study, 28% of patients had indeterminate or unrecognized pancreatic masses on CT clarified with FDG PET, while again demonstrating higher sensitivity and specificity than CT in correctly diagnosing pancreatic carcinoma (92% and 85% vs. 65% and 62%) (32). Additionally, this study showed that the sensitivity of CT imaging improves with the size of the lesion, but the sensitivity of FDG PET is not as dependent on lesion size (32). However, such findings are at variance with the principles of physics, and certainly at very small lesion sizes, both PET and CT would be expected to have diminished performance simply due to impaired signal detection. A recent review by Pakzad et al. (33) suggested that the overall detection sensitivity for PET at diagnosis varies between 90% and 95% and specificity from 82% to 100%, whereas for staging, sensitivity data vary from 61% to 100% and specificity data from 67% to 100% for PET.
Based on the then available data, the European Consensus Conference designated FDG PET as an established indication for differentiation of benign and malignant pancreatic masses (34). However, it is important to keep in mind that these studies do not reflect current clinical practice as PET/CT has rapidly replaced PET at most institutions. Further, uses in Europe are not uniform by country or provider.
There is evidence that the degree of FDG uptake has prognostic value. Nakata et al. (35) noted a correlation between standard uptake value (SUV) and survival in 14 patients with pancreatic adenocarcinoma. Patients with an SUV higher than 3.0 had a mean survival of 5 months, compared with 14 months in those with an SUV lower than 3.0. In a multivariate analysis of 52 patients with pancreatic carcinoma (36), the median survival of patients with an SUV higher than 6.1 was 5 months, compared with 9 months for patients with an SUV lower than 6.1.
Limitations of Fluorodeoxyglucose PET Imaging
As with any imaging modality, FDG PET has limitations in the evaluation of pancreatic cancer. The high incidence of glucose intolerance and diabetes exhibited by patients with pancreatic pathology represents a potential limitation of this modality in the diagnosis of pancreatic cancer. Elevated serum glucose levels result in decreased FDG uptake in tumors due to competitive inhibition; low SUVs with false-negative FDG PET scans have been noted in hyperglycemic patients. Several studies have reported a lower sensitivity in hyperglycemic compared with euglycemic patients (24,28,31). For example, in a study of 106 patients with a disease prevalence of 70% (28), FDG PET had a sensitivity of 98% in a subgroup of euglycemic patients versus 63% in hyperglycemic patients. This has led some investigators to suggest that the SUV be corrected according to serum glucose level (37,38,39,40,41). Although a study of 86 pancreatic lesions by Koyama et al. (41) demonstrated only modestly improved sensitivity and negative predictive value of serum glucose corrected SUV compared to uncorrected SUV (91% and 73% vs. 89% and 70%).
In the studies of Delbeke et al. (31) and Diederichs et al. (40), the presence of elevated serum glucose levels and/or diabetes mellitus may have contributed to false-negative interpretations; but correction of the SUV for serum glucose level has not significantly improved the sensitivity of FDG PET in the diagnosis of pancreatic carcinoma. The true impact of serum glucose levels on the accuracy of FDG PET in pancreatic cancer and other neoplasms remains somewhat controversial, although it is clear that elevated serum glucose levels can reduce tumor FDG uptake significantly.
False-negative study results may also occur when the tumor diameter is less than 1 cm (i.e., small ampullary carcinoma). Ampullary carcinomas arise from the ampulla of Vater and have a better prognosis than pancreatic carcinoma because they cause biliary obstruction and are diagnosed earlier in the course of the disease. The detection rate with FDG imaging is only 70% to 80%, probably because of their smaller size at the time of clinical presentation. In addition, such lesions can be located immediately adjoining bowel, which can be FDG avid, thus lowering tumor/background uptake ratios.
Both glucose and FDG are substrates for cellular mediators of inflammation. Some benign inflammatory lesions, including chronic and acute pancreatitis, can accumulate FDG and result in false-positive interpretations on PET images (24,27,42,43). However, Koyama et al. (41) reported significant differences in SUV of benign and malignant lesions by using an SUV cutoff of 2.1. Even better results were reported by Nakamoto et al. (44) in a study of 49 patients by obtaining an additional 2-hour delayed scan, showing diagnostic accuracy of 91.5% compared to 83% for standard protocol in differentiating malignant and benign lesions of the pancreas. Nakamoto et al. (44) also documented that SUVs of malignant lesions increase over time in comparison with those of benign pancreatic lesions after injection of FDG. Similar results were reported by Nitzsche et al. (45), who performed kinetic analysis of FDG uptake in normal patients and patients with acute pancreatitis, chronic pancreatitis, or pancreatic cancer. Recently, fairly high accuracy was observed in the differentiation of chronic pancreatitis from cancer. In 87% (67 of 77) of the patients with chronic pancreatitis, pancreatic FDG accumulation was absent. Six patients had significant accumulation of FDG, most likely due to active inflammation. In patients with pancreatic cancer, 24 of 26 patients had a positive PET scan. In practice, cancers are usually focal, while pancreatitis is usually diffuse. In some instances, it can be impossible to tell the two conditions apart, especially where there is, for example, mass-forming pancreatitis present.
FDG PET imaging is complementary to CT in the evaluation of patients with pancreatic masses or in whom the diagnosis of pancreatic carcinoma is suspected. In view of the probable decreased sensitivity seen in patients with hyperglycemia, the acquisition of PET images should be performed under controlled metabolic conditions and in the absence of acute inflammatory abdominal disease. If FDG PET findings are equivocal, measurement of FDG kinetics and or delayed images can be performed to differentiate between benign and malignant processes. In practice, parametric imaging is feasible only when dynamic image data are collected; thus, this can usually only be done prospectively. Close correlation with anatomic images is, of course, required for optimal imaging accuracy.
Fluorodeoxyglucose PET for Staging Pancreatic Carcinoma
Pancreatic adenocarcinoma is staged by the tumor, node, metastases (TNM) system (Tables 8.15.1 and 8.15.2). The resectability of a local tumor is determined by whether the tumor extends into major arterial structures and whether long segment involvement, or occlusion, of major venous structures is present (46). TNM classification has recently been updated to reflect advances in surgical technique permitting venous interposition grafts. Local extent of the tumor can only be evaluated with anatomic imaging modalities, which demonstrate the relationship between the tumor, adjacent organs, and vascular structures. Functional imaging modalities cannot replace anatomic imaging in the assessment of local tumor resectability. Even fusion imaging with FDG PET/CT may not significantly improve local staging given the limited intrinsic PET resolution, but this has not been fully resolved. PET can certainly help identify additional foci of disease that may help define resectability.
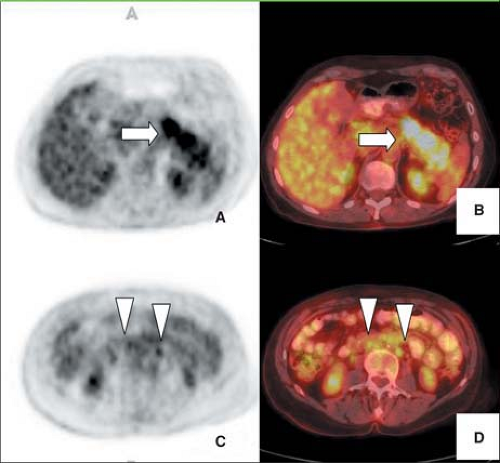
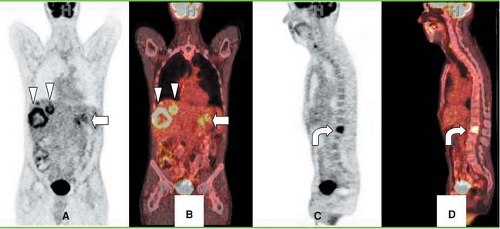
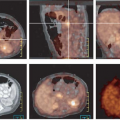
Sixty percent of patients presenting with pancreatic adenocarcinoma have advanced disease (47) (Figs. 8.15.2 and 8.15.3). Detection of nodal and peritoneal disease remains a challenge with all imaging modalities. FDG imaging is not consistently superior to helical CT for node staging but is more accurate than CT for metastases staging—but this area warrants further study with PET/CT. Reported FDG PET sensitivity and specificity for lymph node staging is 49% and 63%, respectively (40). In a study by Delbeke et al. (31), metastases were diagnosed both on CT and on PET in 10 of 21 patients with stage IV disease, but PET demonstrated hepatic metastases not identified or equivocal on CT and/or distant metastases unsuspected clinically in seven additional patients (33%). In four patients (19%), neither CT nor PET imaging showed evidence of metastases, but surgical exploration revealed carcinomatosis in three and a small liver metastasis in one patient.
Table 8.15.1 Tumor, Node, Metastases Classification for the Staging of Pancreatic Cancer | ||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||
FDG PET is sensitive for detection of hepatic metastases, but false-positive findings have been reported in the livers of patients with dilated bile ducts and inflammatory granulomas (48). In a study by Diederichs et al. (40) FDG PET demonstrated sensitivity of 70% and a specificity of 95% for liver metastases, with sensitivity decreasing as lesion size decreased. A direct comparison of PET and MRI with hepatocyte iron contrast showed the MRI technique to be more sensitive, especially for small hepatic lesions, than was PET/CT. On a per patient basis, MRI and FDG PET were comparable in performance, with sensitivities of 96.6% and 93.3%, and accuracies of 97.1% and 85.3%, respectively. However, per-lesion analysis showed MRI to have a higher sensitivity than FDG PET at 81.4% and 67.0%, respectively. PET detected only about 40% of the lesions seen by MRI that were less than 1 cm in diameter. Although MRI liver specific contrast is not yet routinely available, these data do indicate that while PET is a sensitive tool for metastatic pancreatic cancer to the liver, the sensitivity is not as high as can probably ultimately be achieved with MRI given the superior resolution of MRI versus PET. In that study, PET did detect extrahepatic lesions missed by MRI, so the techniques are clearly complementary.
Table 8.15.2 Tumor, Node, Metastases Staging of Pancreatic Cancer | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||
Impact of Fluorodeoxyglucose PET on the Management of Patients with Pancreatic Carcinoma
The rate at which FDG PET may lead to alterations in clinical management clearly depends on the specific therapeutic philosophy employed by the evaluating surgeon. In the study of Delbeke et al. (31), the surgeons advocated pancreaticoduodenectomy only for those patients with potentially curable pancreatic cancer. They also took an aggressive approach to resection, including en bloc retroperitoneal lymphadenectomy and selective resection of the superior mesenteric-portal vein confluence when necessary. Most patients with nonmalignant biliary strictures were managed without resection. In that series of 65 patients, the application of FDG PET imaging, in addition to CT, altered the surgical management in 41% of the patients, 27% by detecting CT-occult pancreatic carcinoma, and 14% by identifying unsuspected distant metastases, or by clarifying the benign nature of lesions equivocal on CT (31).
Fluorodeoxyglucose PET for Detection of Recurrent Pancreatic Carcinoma
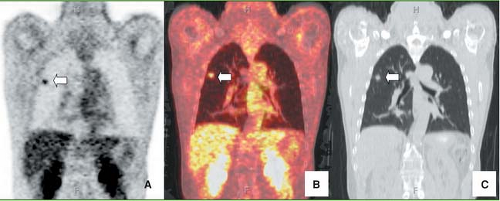
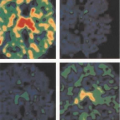
FDG PET utility for monitoring of patients with pancreatic adenocarcinoma after surgical therapy, chemotherapy, and radiation has been confirmed in several studies (32,50,51) (Fig. 8.15.4). For example, Ruf et al. (50) evaluated 39 patients with subsequently
confirmed recurrence after surgery, demonstrating that FDG PET reliably detected local and nonlocoregional recurrences, whereas CT-MRI was more sensitive for the detection of hepatic metastases. Of 25 patients with local recurrences on follow-up, initial imaging suggested relapse in 23 patients. Of these, FDG PET detected 96% (22 of 23) and CT-MRI 39% (9 of 23). Among 12 liver metastases, FDG PET detected 42% (5 of 12). CT-MRI detected 92% (11 of 12) correctly. Moreover, seven of nine abdominal lesions were malignant on follow-up, of which FDG PET detected seven of seven and CT-MRI detected none. Additionally, FDG PET detected extra-abdominal metastases in two patients.
confirmed recurrence after surgery, demonstrating that FDG PET reliably detected local and nonlocoregional recurrences, whereas CT-MRI was more sensitive for the detection of hepatic metastases. Of 25 patients with local recurrences on follow-up, initial imaging suggested relapse in 23 patients. Of these, FDG PET detected 96% (22 of 23) and CT-MRI 39% (9 of 23). Among 12 liver metastases, FDG PET detected 42% (5 of 12). CT-MRI detected 92% (11 of 12) correctly. Moreover, seven of nine abdominal lesions were malignant on follow-up, of which FDG PET detected seven of seven and CT-MRI detected none. Additionally, FDG PET detected extra-abdominal metastases in two patients.
Another study of 19 patients concluded that FDG PET added important incremental information in 50% of the patients, resulting in a change of therapeutic procedure (49). This included
patients with elevated serum tumor marker levels but no findings on anatomic imaging. Therefore, FDG PET may be particularly useful when (a) CT identifies an indistinct region of change in the bed of the resected pancreas that is difficult to differentiate from postoperative or postradiation fibrosis, (b) for the evaluation of new hepatic lesions that may be too small to biopsy, and (c) in patients with rising serum tumor marker levels and a negative conventional work-up. Given the poor prognosis of pancreatic cancer, it is not expected that large differences in patient outcome will result from such studies. However, it is probable that patients will benefit by giving the most appropriate treatment while eliminating treatments viewed as unlikely to be effective. The presentation of recurrent tumor may overlap substantially with that of pancreatic insufficiency or radiation enteritis, and accurate diagnosis is essential given the markedly different treatment approaches.
patients with elevated serum tumor marker levels but no findings on anatomic imaging. Therefore, FDG PET may be particularly useful when (a) CT identifies an indistinct region of change in the bed of the resected pancreas that is difficult to differentiate from postoperative or postradiation fibrosis, (b) for the evaluation of new hepatic lesions that may be too small to biopsy, and (c) in patients with rising serum tumor marker levels and a negative conventional work-up. Given the poor prognosis of pancreatic cancer, it is not expected that large differences in patient outcome will result from such studies. However, it is probable that patients will benefit by giving the most appropriate treatment while eliminating treatments viewed as unlikely to be effective. The presentation of recurrent tumor may overlap substantially with that of pancreatic insufficiency or radiation enteritis, and accurate diagnosis is essential given the markedly different treatment approaches.
Fluorodeoxyglucose PET for Monitoring Therapy
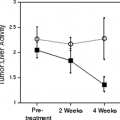
In a study by Higashi et al. (51), FDG PET was useful in monitoring patients after intraoperative radiotherapy for unresectable pancreatic cancer, because the decrease in metabolism in pancreatic cancer could be detected earlier than the decrease in tumor size (Fig. 8.15.5).
A pilot study by Rose et al. (32), determined that FDG PET imaging might be useful for the assessment of tumor response to neoadjuvant therapy and the evaluation of suspected recurrent disease after resection. Nine patients underwent FDG PET imaging before and after neoadjuvant chemoradiation therapy. FDG PET successfully predicted histologic evidence of chemoradiation-induced tumor necrosis in the four patients who demonstrated at least a 50% reduction in tumor SUV after chemoradiation. Among these patients, none showed a measurable reduction in tumor diameter, as assessed by CT. This is consistent with past data that showed a more rapid drop in tumor glucose metabolism levels than the changes in tumor size. The four patients who had FDG PET evidence of tumor response went on to successful resection, all showing 20% to 80% tumor necrosis in the resected specimen. Three patients showed stable FDG uptake and two showed increasing FDG uptake indicative of tumor progression. Among the two patients with progressive disease demonstrated by FDG PET, one showed tumor progression on CT and the other demonstrated stable disease. Among the five patients who showed no response by FDG PET, the disease could be subsequently resected in only two, and only one patient who underwent resection showed evidence of chemoradiation-induced necrosis in the resected specimen.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree