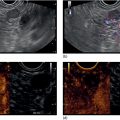
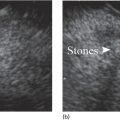
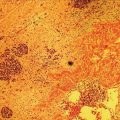
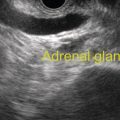
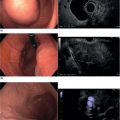
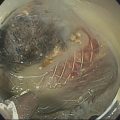
Larissa Fujii‐Lau1, Michael J. Levy2, and Suresh T. Chari3 1 University of Hawaii, Honolulu, HI, USA 2 Mayo Clinic, Rochester, MN, USA 3 MD Anderson Cancer Hospital, Houston, TX, USA Endoscopic ultrasound (EUS) is routinely employed in the evaluation of solid pancreatic masses as it enhances diagnostic accuracy, guides management, and improves patient outcomes. Much has been published regarding the use of EUS for pancreatic adenocarcinoma, which accounts for approximately 95% of all solid pancreatic malignancies. There are fewer EUS data available for endocrine tumors, and even less for lymphoma, solid pseudopapillary tumors, acinar cell carcinoma, and secondary metastatic tumors. Endocrine pancreatic tumors (EPTs) are rare, with an incidence of less than 1 in 100 000. Because of more widespread use and better‐quality cross‐sectional imaging, EPTs are being incidentally identified more commonly. EUS has been shown to be more sensitive at identifying EPTs than cross‐sectional imaging, particularly since a significant proportion of these tumors are less than 20 mm in size. The majority of EPTs (70–90%) are nonfunctioning, followed by gastrinomas and insulinomas; glucagonomas, somatostatinomas, and VIPomas are less often reported. EUS is often performed to assist with preoperative localization of these tumors to help guide surgical resection, which offers the only chance for cure and is usually recommended due to the malignant potential of EPTs. Endoscopic ultrasound localizes 75–95% of insulinomas; the high EUS detection rate for insulinomas is likely explained by the fact that 99% of insulinomas are contained within the pancreas. Approximately 75–100% of pancreatic gastrinomas are identified by EUS compared with only 0–67% of duodenal gastrinomas. The EUS appearance of EPTs is similar regardless of the tumor type or functional status, typically appearing as round, well‐delineated, homogeneous, mildly hypoechoic lesions, often hypervascular with a surrounding hyperechoic rim. They may also appear isoechoic or hyperechoic, have a hypoechoic border, be cystic, and contain calcifications. The EUS technique for localizing these tumors is identical to that of adenocarcinoma, except that a more deliberate exam may be needed to find these often small lesions. The parapancreatic region should also be carefully examined to search for lymphadenopathy and because the primary tumor may be attached by a pedicle or completely separate from the pancreas, which makes it more difficult to locate than intrapancreatic tumors. EUS characteristics may also predict the malignant potential, which can be otherwise difficult to discern in the absence of extensive local invasion or distant metastasis. The presence of anechoic regions, an irregular central echogenic area, or pancreatic duct obstruction corresponds with severe hemorrhage, necrosis, or hyaline degeneration, each of which suggests a malignant tumor, although their predictive value is uncertain. If contrast harmonic EUS is performed, a more heterogeneous enhancement has been shown to correlate to more aggressive tumors (defined as G3 tumors or G1/G2 tumors with morphologic and/or histologic findings of metastatic disease). Endoscopic ultrasound‐guided fine needle aspiration (EUS‐FNA) or fine needle biopsy (EUS‐FNB) helps differentiate benign parapancreatic lymph nodes from primary EPTs, a distinction that can be difficult, especially for insulinomas. Samples obtained by EUS‐FNB can also be tested for Ki‐67 labeling index which is included in the World Health Organization (WHO) 2017 grading system for EPTs. There have been case series on EUS‐guided fine needle injection (EUS‐FNI) of alcohol or radiofrequency ablation for treatment of nonfunctioning EPTs or insulinomas. Localized EUS‐guided treatment of these lesions may be considered in patients with small tumors who are not surgical candidates. However, this should only be performed in tertiary referral centers that have a study protocol in place. More studies are required to determine the optimal technique, injectate volume, and follow‐up of EUS‐guided treatment. Figure 23.1 Images obtained from a patient with a gastrinoma following helical computed tomography (CT) that revealed a pancreatic cyst that was not felt to represent a gastrinoma. Linear endoscopic ultrasound identified malignant vascular thrombosis as highlighted with the use of power Doppler (a). A large endocrine tumor was identified, which appeared more hypoechoic, irregular, and infiltrating than usual. This mass encompassed most of the pancreatic body and tail (b). The tumor also contained a pancreatic tail cystic component that was seen on CT (c). Source: Mayo Clinic. Figure 23.2 Following a negative computed tomography (CT) to search for a presumed insulinoma, linear endoscopic ultrasound identified the insulinoma within the pancreatic body, allowing curative surgical intervention. Source: Mayo Clinic. Approximately one‐third of patients with non‐Hodgkin’s lymphoma (NHL) develop secondary pancreatic involvement. Primary pancreatic lymphoma is rare and accounts for fewer than 3% of extranodal NHL, and less than 0.5% of all pancreatic malignancies. It is generally treated with chemotherapy and/or radiation therapy, commonly resulting in disease remission and occasionally cure. Most tumors appear by EUS as a mildly hypoechoic, solid and less often cystic mass that may be single or multiple. It may be difficult to distinguish primary pancreatic masses from commonly accompanying prominent peripancreatic lymphadenopathy. Although the appearance may mimic adenocarcinomas, lymphomas are less hypoechoic, have less pronounced peritumoral inflammatory changes, and less commonly have vascular invasion and associated pancreatic duct dilatation. Clinical presentation is of little utility as typical “B‐type” symptoms, including fever and night sweats, are seldom present. NHL may be diagnosed by EUS‐FNA with immunophenotyping by flow cytometry. It is important to have a high suspicion for NHL as several EUS‐FNA passes should be placed in a different container specifically for flow cytometry. The diagnostic accuracy may be further improved by obtaining EUS‐FNB to further enhance morphologic and architectural assessment. Figure 23.3 Linear endoscopic ultrasound imaging of a patient with a pancreatic lymphoma. Source: Mayo Clinic. Solid pseudopapillary tumors (SPTs) are rare pancreatic neoplasms that typically develop in young women in the second or third decade of life. They are usually discovered incidentally and typically follow an indolent course. As they can be malignant and develop distant metastases, surgery is the therapy of choice providing curative resection in 95% of patients. Most favor an aggressive resection for malignant disease that includes major venous and even arterial structures and distant metastases. Of note, nodal metastases are unusual and formal lymphadenectomy is not necessary. Continued surveillance of all patients is recommended, and should metastases appear, surgical resection is indicated. Imaging typically reveals large, well‐circumscribed, hypoechoic lesions that may be solid to nearly entirely cystic often containing multiple cystic compartments. EUS‐FNA permits diagnosis in most patients; however, the cytology may be confused with endocrine tumors and acinar cell carcinoma. Negative chromogranin and synaptophysin staining exclude an islet cell neoplasm, whereas a negative keratin stain excludes acinar carcinoma. Immunohistochemical analysis of SPTs usually shows strong staining for CD56 and vimentin and β‐catenin gene mutations are common. Figure 23.4 Endoscopic ultrasound revealed a large well‐circumscribed hypoechoic lesion that contained solid and cystic components with the diagnosis of a solid pseudopapillary tumor established by endoscopic ultrasound‐guided fine needle aspiration (EUS‐FNA) and later confirmed at surgery. Source: Mayo Clinic. Figure 23.5 A pancreatic head acinar cell tumor is identified by endoscopic ultrasound and confirmed at fine needle aspiration (FNA) leading to pancreaticoduodenectomy. Source: Mayo Clinic. Although acinar cells comprise over 80% of all pancreatic cells, acinar cell carcinoma (ACC) accounts for less than 1% of all pancreatic neoplasms. They typically develop in males during the sixth or seventh decade of life. Most patients present with large tumors with associated pain and weight loss. Patients may develop lipase hypersecretion syndrome resulting from systemic secretion of pancreatic enzymes and characterized by fever, polyarthritis, subcutaneous fat necrosis, and eosinophilia. There is some debate as to the clinical course of ACC. While some report poor outcomes, most recent studies have found a better prognosis than for pancreatic adenocarcinoma. Despite the typically large size of ACCs, resection margins are usually negative. Surgical resection is the therapy of choice and while nearly all succumb to their disease, the postoperative survival duration is greater (approximately four to five years) than for pancreatic adenocarcinoma. Neoadjuvant therapy may provide effective downstaging and allow resection in a small cohort of patients. EUS characteristics of ACC are nonspecific. Most are hypoechoic but ACC can also be isoechoic or slightly hyperechoic. There may be cystic components, calcifications, diffuse pancreatic enlargement, or pancreatic duct dilation present. EUS elastography has not been shown to help differentiate ACC from pancreatic adenocarcinoma. However, contrast harmonic EUS has different enhancement properties and ACC more often had a gradual enhancement pattern as compared to the hypoenhancement seen with pancreatic adenocarcinomas. Immunohistochemical analysis of ACC usually shows strong staining for trypsin, chymotrypsin, and lipase, permitting diagnosis. The most common neoplasm in the differential diagnosis of patients with ACC is well‐differentiated EPT. Immunohistochemical stains for the neuroendocrine markers synaptophysin and chromogranin help differentiate these neoplasms. However, mixed acinar–endocrine neoplasms occur and are associated with improved prognosis over pure ACC. Pancreatic metastases account for approximately 1% of all resected solid pancreatic masses. While over 30 sites of origin have been reported, the most common primary tumors include renal cell carcinoma, lung, breast, colon, melanoma, sarcoma, and ovarian. These tumors are notable for a prolonged delay from initial primary tumor diagnosis until pancreatic metastasis that averages 5–10 years and may occur more than 25 years later. While most metastasize to the pancreatic head, multiple metastases are seen in one‐third to half of patients, and diffuse infiltration is also reported. Pancreatic metastases are more often resectable and portend a better prognosis than patients with primary pancreatic adenocarcinoma. Imaging can vary tremendously for these lesions that may be solid and/or cystic, have variable echodensity, and are often hypervascular. EUS‐FNA can usually establish the diagnosis, but EUS‐FNB and immunostaining may be necessary for definitive diagnosis. Endoscopic ultrasound further impacts patient care through the detection of multifocal disease undetected by noninvasive imaging. Endosonographers should suspect metastatic tumor in patients with a prior diagnosis of distant neoplasia given the relatively indolent course and improved surgical outcomes with postoperative survival of two to four years. Endoscopic ultrasound is routinely used to evaluate pancreatic masses. By offering high‐resolution imaging and tissue sampling, EUS can often establish the malignant potential of these masses, enhance staging accuracy, and determine the tumor type to help guide treatment and improve outcomes. Adequate tissue obtained during EUS‐FNA or EUS‐FNB is required for immunohistologic staining to help differentiate these lesions.
23
Pancreatic Malignancy (Non‐adenocarcinoma)
Introduction
Endocrine pancreatic tumors (Figures 23.1 and 23.2)


Primary pancreatic lymphoma (Figure 23.3)

Solid pseudopapillary tumors (Figure 23.4)


Acinar cell carcinoma (Figure 23.5)
Secondary metastatic tumors (Figure 23.6)
Summary
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree