Anatomy, embryology, pathophysiology
- ◼
The pancreas is a lobulated, unencapsulated gland located in the anterior pararenal space of the retroperitoneum.
- ◼
Pancreatic development: two outpouchings or buds develop from the endodermal lining of the duodenum. The ventral bud will eventually form the posterior pancreatic head and uncinate process whereas the dorsal bud will form the anterior pancreatic head, body, and tail. The pancreatic buds have their own ductal system and fuse through a complex process. In the final configuration, the dorsal pancreatic duct is connected to the ventral pancreatic duct via the duct of Wirsung, which empties into the major papilla. The remnant dorsal duct is called the duct of Santorini and empties into the minor papilla.
- ◼
The pancreas is divided into four regions: head, neck, body, and tail. The head is situated in the duodenal C-loop, to the right of the superior mesenteric vein. The uncinate process is the caudal extension of the pancreatic head. The body lies between the left border of the superior mesenteric vein and the left border of the aorta. The pancreatic tail is from the left border of the aorta to the splenic hilum.
Techniques
- ◼
Computed tomography (CT): given its practicality, CT is the initial imaging modality of choice for overall morphological assessment of acute and chronic pancreatitis.
- ◼
Ultrasonography (US): essential to assess for gallstones and for secondary findings of choledocholithiasis.
- ◼
Magnetic resonance imaging (MRI): MRI has superior soft tissue contrast compared with CT and US. Magnetic resonance cholangiopancreatography (MRCP) is sensitive and specific for detecting stones in the biliary tree, especially of the proximal common bile duct, which may not be seen on ultrasound. MRI also allows for visualization of the entire pancreatic duct with improved sensitivity for abnormalities with secretin administration.
Protocols
Dynamic computed tomography
- ◼
Early arterial phase: acquired around 20 seconds after intravenous (IV) contrast administration. The contrast should preferentially concentrate within the arterial tree with almost no enhancement of the pancreatic parenchyma. This phase is useful to evaluate for vascular complications.
- ◼
Delayed arterial phase or pancreatic phase: acquired around 45 seconds after the injection of IV contrast. Pancreatic parenchyma should enhance optimally during this phase.
- ◼
Portal venous phase: acquired around 75 seconds after injection of IV contrast. The pancreas should demonstrate homogeneous increase in attenuation by 100 to 150 HU. In the absence of necrosis, the pancreas and spleen should be similar in attenuation on this phase.
- ◼
Unenhanced CT could be added to the imaging protocol if there is high suspicion for hemorrhage.
Magnetic resonance imaging
T1-weighted imaging
- ◼
The pancreas is intrinsically hyperintense on T1-weighted images because of high quantity of aqueous proteins within the gland, presence of large quantities of endoplasmic reticula in the acinar cells, and paramagnetic ions, such as manganese.
- ◼
T1-weighted gradient recalled echo (GRE) images are used for dynamic imaging of the pancreas after IV administration of gadolinium. Dynamic images can be acquired at around 25 seconds (early arterial phase), around 45 seconds (pancreatic phase), around 80 seconds (portal venous phase), and 5 minutes (equilibrium phase).
T2-weighted imaging
- ◼
MRCP is a heavily T2-weighted pulse sequence to selectively display slow-moving fluid-filled structures as areas of high intensity. It is acquired using fast recovery sequences or steady-state free precession with suspended respiration. The data is processed using maximum intensity projection to display the pancreatic duct and biliary tree. It is highly sensitive and specific for diagnosing biliary duct dilatation and choledocholithiasis.
- ◼
MRCP can also demonstrate duct disruption, leakage, duct communication with a pancreatic pseudocyst, and structural abnormalities, such as divisum and anomalous pancreaticobiliary junction, which can cause recurrent attacks of pancreatitis.
- ◼
Secretin can be given to improve visualization of the main pancreatic duct and side branches. Rapid imaging after secretin administration gives information on the main pancreatic duct flow dynamics.
Specific magnetic resonance imaging protocols
- ◼
Axial T1-weighted three-dimensional (3D) two-point Dixon GRE sequence with water-only and fat-only images, as well as in-phase and out-of-phase images.
- ◼
Dynamic T1-weighted images with 3D GRE with fat suppression (precontrast, arterial, portal venous, and 5-minute delayed venous phase).
- ◼
T2-weighted, fat suppressed images (axial).
- ◼
T2-weighted, fast spin echo (FSE) images without fat suppression (axial and coronal).
- ◼
Two-dimensional (2D) MRCP generated with 40-mm thick, eight paracoronal projections to visualize the pancreatic duct from different angles.
- ◼
3D MRCP: 2 to 3 mm slick thickness with 3D FSE sequences with respiratory gating or breathe holds. Source images are postprocessed into maximum intensity projection images.
Specific disease processes
Acute pancreatitis
- ◼
The inflammatory process in acute pancreatitis is triggered by the premature activation of pancreatic enzymes, with resultant autodigestion of the pancreatic parenchyma. The inflammatory process may remain localized to the pancreas and regional tissues or trigger a systemic inflammatory response and multisystem organ failure.
- ◼
The most common causes of acute pancreatitis are gallstones (45%) and alcohol (35%). Almost 20% of cases are idiopathic.
- ◼
Pathophysiology of acute pancreatitis is subdivided into acute interstitial edematous pancreatitis and necrotizing pancreatitis.
- ◼
Interstitial edematous pancreatitis: The entire pancreas will enhance on contrast-enhanced CT or MRI. In mild, self-limiting cases of pancreatitis, the pancreas will appear normal on imaging. In more severe cases, the pancreas may be focally or diffusely edematous with peripancreatic inflammation or fluid ( Fig. 12.1 ).

Fig. 12.1
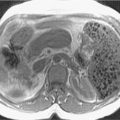
Acute edematous interstitial pancreatitis. A, Axial contrast-enhanced computed tomography (CT) image showing a diffusely enlarged pancreas and peripancreatic inflammatory fat stranding. B, Axial CT image of another patient with mild pancreatitis showing an acute fluid collection inferior to the pancreas. Note that, unlike a pseudocyst, acute fluid collections do not have a well-defined wall.
(From Sahani DV, Samir AE. Abdominal Imaging , ed 2. Philadelphia: Elsevier; 2017.)
- ◼
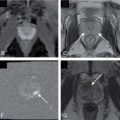
Necrotizing pancreatitis: This term applies when there is focal or diffuse nonenhancement of the pancreatic parenchyma or peripancreatic tissue or, most commonly, both ( Figs. 12.2–12.4 ). Because necrotic pancreatic parenchyma becomes more defined 2 to 3 days after the onset of symptoms, a contrast-enhanced CT performed 48 to 72 hours after the onset of an acute attack may give more reliable information.

Fig. 12.4
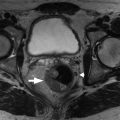
A and B, Gadolinium-enhanced T1-weighted gradient echo images show lack of enhancement of the head and body of the pancreas ( arrowheads ) suggesting the presence of necrosis. Contrast-enhanced magnetic resonance imaging is equivalent to computed tomography for the demonstration of pancreatic necrosis.
(From Sahani DV, Samir AE. Abdominal Imaging , ed 2. Philadelphia: Elsevier; 2017.)

Fig. 12.3
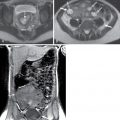
Acute pancreatitis with peripancreatic fat necrosis. Collection in the peripancreatic tissue with islands of fat ( arrowhead ) suggests fat necrosis.
(From Sahani DV, Samir AE. Abdominal Imaging , ed 2. Philadelphia: Elsevier; 2017.)

Fig. 12.2
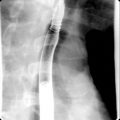
Acute necrotizing pancreatitis. Axial contrast-enhanced computed tomography image demonstrating nonenhancement of the distal body and tail of the pancreas, suggesting necrosis. Note that the head and proximal body of the pancreas demonstrate normal enhancement.
(From Sahani DV, Samir AE. Abdominal Imaging, ed 2. Philadelphia: Elsevier; 2017.)
- ◼
The 2012 revised Atlanta classification standardizes terminology of different types of fluid collections and complications in acute pancreatitis. The most important factors to categorize the collections are: 1) time course (≤4 weeks or >4 weeks from onset of symptoms) and 2) presence or absence of necrosis.
- ◼
Acute peripancreatic fluid collection: seen within 4 weeks of pain in the setting of acute interstitial edematous pancreatitis. Imaging demonstrates a homogeneous, fluid-attenuating collection that occurs in the vicinity of the pancreas or dissects into the lesser sac, anterior pararenal spaces, transverse mesocolon, or mesenteric root.
- ◼
Pseudocyst: after 4 weeks, if an acute peripancreatic fluid collection has not resolved, it is classified as a pseudocyst ( Fig. 12.5 ).
- ◼