Part 2 Procedural Cases
Case 21
Clinical History
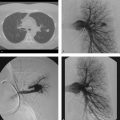
A 61-year-old man with buttock claudication, absent femoral pulses, and impotence (Fig. 21‑1 ).

Key Finding
Treatment options for Leriche’s syndrome defined as buttock claudication, absent femoral pulses, and impotence.
Top 3 Treatment Approaches
Percutaneous transluminal angioplasty (PTA). Angioplasty is best reserved for patients with short-segment stenotic disease of the distal aorta or iliac arteries (e.g., Trans-Atlantic Inter-Society Consensus [TASC] II type A and B lesions). Angioplasty has similar, good results in short-segment distal aortic lesions when compared with stent placement. In the iliac arteries, there is conflicting data, some denote similar patency rates between angioplasty and stent placement, while others suggest about a 5 to 10% greater patency in the stent population.
Percutaneous stent placement. Similar patient and lesion morphology as angioplasty is appropriate for stent placement. Most operators will place stents in the setting of failed angioplasty, densely calcified lesions, or in short-segment occlusions. Technical success is noted in about 99% of cases, with excellent primary patency rates—93 and 86% at 5 and 10 years, respectively.
Open surgery (e.g., aortobifemoral bypass [ABF]). Surgery becomes the preferred treatment approach when lesion morphology includes complete occlusion of the distal abdominal aorta extending into the common iliac arteries (e.g., TASC II type C and D lesions). ABF has been shown to have 90 and 75% patency rates at 5 and 10 years, respectively. When unable to perform ABF, extraanatomic bypasses (e.g., axillary-bifemoral) have good success with 75% patency rates at 5 years.
Additional Therapeutic Consideration
Medical management: All patients should have risk factor modification to include smoking cessation, management of hypertension and hyperlipidemia. Diabetes control, where applicable, is important, too. Aspirin and other antiplatelet agents can improve symptoms, and is important in decreasing adverse coronary and cerebrovascular events. Medical management alone is appropriate for asymptomatic patients or those with mild claudication who have not tried cilostazol or an exercise/walking program.
Diagnosis
Bilateral common iliac artery stenosis treated with kissing stents.
Pearls
Classic Leriche’s syndrome patients are middle-aged male smokers with a history of elevated cholesterol.
Computed tomography angiography (CTA) or magnetic resonance angiography (MRA) is important in procedural planning—determination of endovascular versus open repair.
TASC guidelines from 2007 classify aortoiliac disease morphology (and other peripheral vascular bed disease morphology) into types A to D.
Patient comorbidities can make open surgical treatments inappropriate, leaving endovascular techniques as the only remaining revascularization options.
Suggested Reading
Neisen MJ. Endovascular management of aortoiliac occlusive disease. Semin Intervent Radiol. 2009; 26(4):296–302Case 22
Clinical History
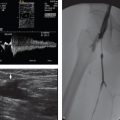
A 27-year-old Asian woman with left arm pain (Fig. 22‑1 ).

Key Finding
Great vessel stenosis.
Top 3 Differential Diagnoses
Atherosclerosis. Atherosclerosis is the most common cause of great vessel stenosis. Multiple vessels are usually involved. Patients are typically older and have risk factors such as hypertension, smoking, hyperlipidemia, and/or diabetes. Concomitant cardiovascular, cerebrovascular, renovascular, and peripheral vascular disease is common. Symptoms are related to the specific end organ being underperfused, and therapy is geared toward relief of said symptoms. Catheter-directed therapies of the great vessels are best due to the decreased morbidity compared with open surgery. Technical success rates exceed 95%. Long-term clinical success of angioplasty for stenosis and stent placement for occlusion ranges from 70 to 90%.
Vasculitis. Great vessel and aortic vasculitis is usually secondary to primary conditions such as Takayasu or giant cell arteritis (GCA). Takayasu tends to affect younger, Asian female patients, and manifests clinically with malaise, arthralgias, and gradual onset of extremity claudication. Clinical examination may reveal asymmetric pulses or blood pressure in the upper extremities. GCA typically is seen in older Caucasian females. Patients have fevers, polymyalgia rheumatica, facial or upper extremity claudication, visual disturbances, blindness, and generalized constitutional symptoms. The diagnosis of GCA is confirmed with temporal artery biopsy. Treatment of vasculitis consists of corticosteroid administration. Once acute flares have resolved, residual stenoses may be treated percutaneously should concordant symptoms persist.
Dissection. Stanford A dissections may have false lumen extension into the origins of the great vessels. This can lead to end-organ hypoperfusion/stroke. Surgical management is the usual treatment. In nonsurgical patients, endoluminal options include stent or stent-graft placement at the leading intimal tear or fenestration of the intimal flap.
Additional Diagnostic Consideration
Radiation: Radiation therapy to the chest wall or neck may lead to multiple, smooth, long-segment strictures of affected vessels. History is the key to making this diagnosis. Treatments are centered on endoluminal options for arch vessel involvement given the high morbidity associated with surgical procedures, along with similar patency rates.
Diagnosis
Vasculitis (Takayasu arteritis).
Pearls
Atherosclerosis is the most common cause of great vessel stenosis and typically affects older individuals.
GCA and Takayasu arteritis are common vasculitides affecting the great vessels; treatment is with steroids.
Aortic dissections may involve the great vessels; Stanford A dissections are typically managed surgically.
Radiation therapy to the neck results in long-segment strictures; endoluminal treatment is preferred.
Suggested Readings
Hadjipetrou P, Cox S, Piemonte T, Eisenhauer A. Percutaneous revascularization of atherosclerotic obstruction of aortic arch vessels. J Am Coll Cardiol. 1999; 33(5):1238–1245 Roane DW, Griger DR. An approach to diagnosis and initial management of systemic vasculitis. Am Fam Physician. 1999; 60(5):1421–1430 Zimpfer D, Czerny M, Kettenbach J, et al. Treatment of acute type a dissection by percutaneous endovascular stent-graft placement. Ann Thorac Surg. 2006; 82(2):747–749Case 23
Clinical History
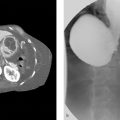
A 62-year-old man with chest pain, hypertension, and systolic murmur auscultated over the right upper sternal border (Fig. 23‑1 ).

Key Finding
Aortic dissection.
Top 3 Differential Diagnoses
Hypertension. Hypertension is the most common cause of aortic dissection and typically occurs in men between 50 and 70 years of age. Poorly controlled hypertension results in intimal tearing which may progress to dissection. Patients present acutely with chest pain which radiates to the back. Involvement of the ascending aorta (Stanford A) requires surgical or endovascular intervention. This is due to the danger of coronary artery involvement leading to myocardial infarction, rapidly increasing aneurysmal enlargement leading to rupture (specifically into the pericardium leading to tamponade) or enlargement leading to aortic valve insufficiency. Dissections involving only the descending aorta (Stanford B) are treated medically in the absence of end-organ ischemia.
Connective tissue disease. Connective tissue diseases, such as Ehlers–Danlos or Marfan’s disease, can affect the aortic media. Most notably, they cause a cystic medial necrosis of the aortic wall. This can lead to enlargement of the aorta, most specifically annuloaortic ectasia, which refers to a proximal dilation of the aortic annulus (at the level of the sinus of Valsalva) and ascending aorta. Dissections or vascular rupture are common presenting signs in the majority of cases. When dissection occurs, it often involves the entire length of the aorta. Arterial rupture may be preceded by aneurysm, arteriovenous fistulae, or dissection, which may be seen with magnetic resonance (MR), computed tomography (CT), or conventional angiography. Alternatively, annuloaortic ectasia can lead to aortic insufficiency leading to congestive heart failure.
Bicuspid aortic valve. Bicuspid aortic valve is a common congenital anomaly which is more common in males. Abnormal valve leaflet morphology results in early degeneration, leading first to stenosis followed in some instances by valve incompetence. Patients with a bicuspid aortic valve are at higher risk of aortic aneurysms and dissection, likely due to poststenotic hemodynamic stresses. Echocardiography, MR, and CT are the best noninvasive methods to evaluate for bicuspid aortic valve.
Additional Diagnostic Considerations
Coarctation of the aorta: Aortic coarctation is a congenital anomaly which results in eccentric narrowing and infolding of the aorta adjacent to the left subclavian artery and stenosis to left ventricular outflow. It may be associated with a bicuspid aortic valve, aortic aneurysms, dissection, and congenital heart disease. Imaging findings include inferior rib notching due to enlarged intercostal arteries providing collateral blood flow. A “figure 3” aortic appearance results from stenosis and dilation of the aorta.
Trauma: Thoracic aortic injury from blunt chest trauma typically occurs at the aortic isthmus, just distal to the left subclavian artery, and at the level of the diaphragm. Aortic root injuries are usually fatal. Trauma can lead to a large number of injury types to the aorta—dissection being one of them.
Diagnosis
Aortic dissection (Stanford A) due to hypertension.
Pearls
Chronic hypertension is the most common causes of aortic dissection.
Stanford A (ascending aorta involved) lesions are managed surgically; Stanford B are managed medically.
Annuloaortic ectasia, bicuspid aortic valve, and aortic coarctation have increased risk of aortic dissection.
Suggested Readings
Kuhlman JE, Pozniak MA, Collins J, Knisely BL. Radiographic and CT findings of blunt chest trauma: aortic injuries and looking beyond them. Radiographics. 1998;18(5):1085-1106, discussion 1107–1108, quiz 1 Tatli S, Yucel EK, Lipton MJ. CT and MR imaging of the thoracic aorta: current techniques and clinical applications. Radiol. Clin North Am. 2004; 42(3):565-585, viCase 24
Clinical History
A 22-year-old restrained passenger in a high-speed automobile accident (Fig. 24‑1 ).

Key Finding
Traumatic aortic injury.
Top 3 Differential Diagnoses
Pseudoaneurysm. The aortic wall is composed of three layers of tissue—intima, composed of endothelium and basement membrane; media, composed of elastic and muscular tissue; and adventitia, composed of thin connective tissue. A tear of both the intima and media results in a pseudoaneurysm—a bulge in the lumen of the aorta contained only by the adventitia or other mediastinal tissues. In patients who survive to receive medical attention, such a tear usually involves only a portion of the aortic circumference. Most aortic injuries occur near the ligamentum arteriosum. Radiographs may reveal an enlarged aortic contour and/or widened mediastinum. Contrast-enhanced computed tomography (CECT) or angiography reveals focal irregularity or bulge of the aortic wall without an intimal flap. Often the injured aorta will be surrounded by hematoma on CT. Risk of pseudoaneurysm rupture is high, though delayed repair is an option in some patients. Management may include percutaneous stent-graft placement in select patients. Surgical graft placement is often necessary due to proximity of the great vessels.
Aortic dissection. When an injury causes a defect in the intima, blood may enter the media of the artery at arterial pressure. The pressurized blood dissects through the tissue of the media, peeling the intima off the wall of the aorta. Radiographs may reveal enlarged aortic contour. CECT and angiography may reveal irregularity of the aortic lumen, enlarged aorta, and an intimal flap. Aortic intimal calcifications may be displaced. Management depends on a number of factors, including the location of the dissection and its relationship to the great vessels. Some traumatic dissections are amenable to percutaneous stent-graft placement, though dissections of the ascending aorta require urgent surgical management.
Intramural hematoma. The aortic media contains vaso vasorum—blood vessels that supply the tissue of the aorta. Injury to these vessels causes bleeding into the wall of the aorta, resulting in an intramural hematoma. The intima is usually intact. Imaging findings include focal hyperdensity within the wall of the aorta on non-CECT (NCECT) and a focally thickened, nonenhancing aortic wall on CECT. Transesophageal echocardiography (TEE) and magnetic resonance imaging (MRI) are more accurate in making the diagnosis, revealing a curvilinear intramural mass with blood products. Angiography often fails to demonstrate the intramural hematoma. Current management is the same as that of aortic dissection, though there is a paucity of clinical trial data.
Additional Diagnostic Consideration
Aortic transection: When all three layers of the aortic wall are circumferentially torn, blood flows freely out of the aorta into the surrounding tissues. Such injuries are nearly always fatal. About 85% of patients with aortic injuries die before reaching the hospital. Emergent surgical repair is the only treatment option for patients who survive long enough to receive medical care.
Diagnosis
Aortic pseudoaneurysm.
Pearls
Pseudoaneurysms are contained ruptures; prompt treatment (surgical or endovascular) is warranted.
Dissections result from intimal injury with true and false lumens; treatment depends on extent of injury.
Intramural hematomas are hyperdense (CT) or hyperintense (MRI) collections within the vessel wall.
Aortic transaction is when all three layers of the aorta are circumferentially torn; it is nearly always fatal.
Suggested Readings
Koenig TR, West OC. Diagnosing acute traumatic aortic injury with computed tomography angiography: signs and potential pitfalls. Curr Probl Diagn Radiol. 2004; 33(3):97–105 Rousseau H, Dambrin C, Marcheix B, et al. Acute traumatic aortic rupture: a comparison of surgical and stent-graft repair. J Thorac Cardiovasc Surg. 2005;129(5):1050–1055Case 25
Clinical History
A 60-year-old woman in motor vehicle accident (Fig. 25‑1 ).

Key Finding
Classification of solid organ injury.
Top 3 Organs Classified
Spleen. The spleen is the most commonly injured abdominal organ in blunt trauma. In general, hemodynamically stable patients are managed nonoperatively, with 12 to 15% failing. Otherwise, combinations of open and endovascular therapy are used, with embolization leading to splenic salvage rates above 85%. Indications for angiography include high-grade splenic injury (> grade III), presence of vascular injury on computed tomography angiography (CTA) (even on lower-grade injuries), clinical evidence of ongoing bleeding, and/or moderate hemoperitoneum. Embolization is either accomplished proximally or distally. Proximal techniques are often used for multiple vascular injuries or high-grade parenchymal injuries, by placing coils or vascular plug just distal to the pancreatic arteries, in order to decrease the arterial pressure to the spleen. Distal technique is typically used with isolated vascular injury or lower-grade splenic injury. This may encompass a range of embolization techniques, from coil embolization of specific intraparenchymal injuries to focal Gelfoam slurry, in hopes of preserving splenic function.
Liver. The liver is the second most commonly injured abdominal organ in blunt trauma. Given the complexity of the organ, injury can affect artery, vein, or biliary duct. About 85% of liver injuries require some type of intervention (usually due to arterial or biliary injury). Indications for angiography include American Association for the Surgery of Trauma (AAST) grade greater than or equal to III, contrast blush on CTA, and those who are only transient responders to resuscitation. Arterial embolization is beneficial in the setting of initial damage control surgery with liver packing, and ongoing bleeding clinically. Success rates of embolization range from 88 to 100% with a definite mortality benefit. Embolization is usually done distally, with coils, glue, or Gelfoam.
Kidney. Renal injury is noted in about 2% of blunt trauma and is rarely isolated (usually associated with spleen or liver injuries). About 80% of renal injuries are minor and managed nonoperatively. Grade II to IV injuries are addressed via a multidisciplinary approach with attempt at renal salvage, while grade V injuries typically lead to nephrectomy. Indications for embolization include contrast blush on imaging, presence of a pseudoaneurysm or arteriovenous fistula, or some hemodynamically unstable patients. Embolization is successful about 95% of the time (as primary management), and is accomplished with supraselective coils, glue, or Gelfoam.
Additional Organs
Adrenal: Indications for embolization are rare, and entertained when active extravasation is noted on CT. There are only a few case reports of this procedure, with no consensus on indications or materials.
Pancreas: Injury to the pancreas is rare, and difficult to diagnose radiographically, often presenting clinically and in a delayed manner. Embolization is reserved for specific, isolated vascular injuries, and the operator must avoid parenchymal embolization due to severe complication of pancreatitis and abscess.
Diagnosis
Grade III splenic injury.
Pearls
CT angiography has largely replaced traditional diagnostic angiography to evaluate vascular injury.
In unstable patients, many trauma centers have utilized embolization as part of primary resuscitation.
If angiography fails to find injury seen on CT, consider provocative maneuvers (e.g., nitro) or prophylactic embolization given the high possibility of vasospasm instead of spontaneous cessation of bleeding.
Suggested Reading
Yamada R, Guimaraes M, Schonholz C. Endovascular Management of Abdominal Solid Organ Trauma. Endovascular Today September 2014: 44–52. http://www.aast.org/Library/TraumaTools/InjuryScoringScales.aspx . Accessed May 1, 2016.Case 26
Clinical History
A 58-year-old woman with bilateral lower extremity claudication and diminished femoral pulses (Fig. 26‑1 ).

Key Finding
Infrarenal aortic occlusion.
Top 3 Differential Diagnoses
Atherosclerosis. This distribution of occlusive disease is most often seen in smokers, presenting with claudication (80%), rest pain (25%), and tissue loss (15%). About 75% of men will be impotent. Preoperative CTA/MRA should be utilized to aid in management decisions. Management depends on symptoms, morphology, and distribution of the occlusive disease, and patient comorbidities. Some are managed with lifestyle modification and medicines, while others are good candidates for endoluminal therapies. Surgical management usually entails aortobifemoral bypass. Operative mortality is around 5%, with a 67% 5-year survival rate.
Thrombus/embolus. Clot may be formed in situ or come from a proximal source (e.g., cardiac). The underlying pathology is extremely important for management purposes. In the setting of a previously placed aortobifemoral bypass graft, the patient may present acutely with recurrence of symptoms. Endoluminal management is preferred and entails thrombolysis with probable perianastomotic angioplasty and/or stent placement. Embolus lodging in a region of aortic tapering may present with acute ischemia, requiring endoluminal thrombolysis or embolectomy (depending on clinical status). Thrombosis of an aneurysm can also occur (usually in hypercoagulable states) and may merit anything from emergent operative intervention to conservative management with surveillance. History and CTA/MRA are important steps in initial management and determination of appropriate therapy.
Vasculitis. Takayasu arteritis can involve the aorta and its branches. Patients tend to be younger than those with atherosclerosis and have systemic clinical findings (e.g., fevers, fatigue, malaise). Management of the vascular lesions should be delayed until the more fibrotic phase of the disease. When the erythrocyte sedimentation rate (ESR) has normalized, the lesions can be handled similarly to atherosclerotic lesions with surgery or endovascular options. Appropriate treatment depends on the morphology of the lesions, as well as the clinical status of the patient (e.g., symptoms and comorbidities). Relapses can occur.
Additional Diagnostic Consideration
Dissection: Dissections may occur as a result of hypertension, trauma, or iatrogenia. Preoperative CTA/MRA helps to make the diagnosis and aids in management decisions. Medical management, endoluminal stent placement or fenestration, or open surgical repair have all been utilized, depending on the patient’s symptoms and comorbidities.
Diagnosis
Atherosclerosis.
Pearls
Atherosclerosis is a common cause of infrarenal aortic occlusion; treatment depends on lesion morphology.
Clot may form in situ (thrombus) or result from proximal sources (embolus), such as cardiac thrombi.
Vasculitis involves younger patients with systemic findings; lesions are treated in the fibrotic phase.
Dissections often occur from hypertension or trauma; treatment depends on symptoms and comorbidities.
Suggested Readings
Kim SH, Jeong JY, Kim YI, Choi YH, Chung JW, Hyung Park J. SCVIR 2002 Film Panel case 3: aortic occlusion secondary to intimal sarcoma. J Vasc Interv Radiol. 2002; 13(5):537–541 Ligush J, Jr, Criado E, Burnham SJ, Johnson G, Jr, Keagy BA. Management and outcome of chronic atherosclerotic infrarenal aortic occlusion. J Vasc Surg. 1996; 24(3):394-404, discussion 404–405 Uberoi R, Tsetis D. Standards for the endovascular management of aortic occlusive disease. Cardiovasc Intervent Radiol. 2007;30:814–819Case 27
Clinical History
A 50-year-old man with cirrhosis (Fig. 27‑1 ).

Key Finding
Arterial vascular changes associated with cirrhosis.
Top 3 Changes
Peripheral corkscrewing of hepatic arteries. Because of decreased portal blood flow, with a resultant increase in hepatic arterial inflow, combined with liver parenchymal fibrosis shortening the length necessary for the artery to travel, the hepatic arteries become enlarged and tortuous in cirrhosis, giving the characteristic corkscrew appearance seen on imaging.
Mottled parenchyma due to fibrosis/distortion of hepatic architecture. In more advanced cirrhosis, fibrosis can appear as focal, usually wedge-shaped tissue radiating from the porta hepatis. Fibrosis is more often seen in segments IV, VII, and VIII, with associated capsular retraction. On contrast injection, the areas of fibrosis are usually hypoenhancing, creating a mottled appearance on the parenchymal phase of imaging. Rarely, these focal areas can be hyperenhancing, which can mimic hepatoma when capsular retraction and the typical wedge shape are absent.
Arterioportal shunts. The distorted architecture of the hepatic parenchyma in advanced cirrhosis is a predisposing condition in the development of arterioportal shunting. On contrast CT, arterioportal shunts present as transient hepatic attenuation differences, which are wedge shaped, subcapsular, and have no mass effect on adjacent vessels, bile ducts, or liver capsule. The communication between the hepatic artery and portal vein adds complexity to endovascular embolization of more distal liver lesions.
Additional Consideration
Aneurysms: Liver cirrhosis is a risk factor for splenic artery aneurysm formation. Other mesenteric aneurysms are less common.
Diagnosis
Cirrhotic arterial vascular changes.
Pearls
Knowledge of these vascular changes improves safety and efficacy in the endovascular treatment of cirrhotic patients with liver cancer.
Coil embolization of arterioportal shunts may be necessary to fully treat liver cancer using transarterial chemoembolization.
Case 28
Clinical History
A 23-year-old man with right leg pain (Fig. 28‑1 ).

Key Finding
Popliteal artery occlusion.
Top 3 Differential Diagnoses
Atherosclerosis. Atherosclerosis is the most common cause of popliteal artery stenosis and occlusion. Risk factors include hypertension, diabetes, smoking, and hyperlipidemia. Endothelial injury leads to a fibrotic plaque that can rupture or calcify, ultimately leading to narrowing and occlusion. Patients may present with claudication, rest pain, or tissue loss. Treatment is geared toward the morphology of the lesion and the symptoms. Medical management focuses on risk factor modification (e.g., hyperlipidemia) or symptom management (e.g., claudication). Interventions include angioplasty, stents/stent grafts, patch angioplasty, or bypass surgery. Overall patient health plays an important role in the choice of intervention.
Embolism. Emboli present acutely with pain or limb threat. Sources include the heart (e.g., arrhythmias) and proximal arterial lesions (e.g., abdominal aortic aneurysm [AAA]). Thrombolysis can be used if time allows versus thrombectomy.
Trauma. Occlusion of the popliteal artery can be seen in blunt (e.g., motor vehicle accident) or penetrating trauma. A high index of suspicion is requisite given possible relocation of the knee joint prior to presentation, as well as preservation of the distal arterial pulse despite injury. Prompt and accurate diagnosis is required to limit morbidity, with angiography being the best examination. Surgical repair is almost always necessary.
Additional Diagnostic Considerations
Thrombosed popliteal artery aneurysm: Aneurysms are usually true (involving all layers) and degenerative (similar risk factors to atherosclerosis). Rarely, aneurysms form from connective tissue disorders, trauma, or vasculitis. Aneurysms are defined in the popliteal artery as focal areas of widening when compared to normal (≥ 8 mm in diameter). Popliteal artery aneurysms are bilateral 50 to 70% of the time and are associated with abdominal aortic aneurysms in 30 to 50% of cases. Patients may present with thrombosis, distal emboli (blue toe syndrome), or rupture (rare). Treatment is usually surgical, but stent grafts have been used in patients at high operative risk. Endovascular thrombolysis is often required.
Popliteal artery entrapment syndrome: Narrowing of the vessel occurs due to an abnormal relationship of the artery to the medial head of the gastrocnemius or popliteus, or sometimes due to muscle hypertrophy. Patients are usually young and athletic, presenting with claudication. In the proper setting, MR or angiography with the foot neutral and in flexion can be diagnostic. Treatment consists of myomectomy. Left untreated, permanent arterial narrowing will usually result.
Cystic adventitial disease: This rare disease consists of mucoid cysts in the adventitia, leading to compression of the artery. Patients are usually middle-aged males with claudication. MRI best characterizes the lesion, showing the narrowing of the vessel and the mucinous cyst. Treatment options include cyst aspiration (with risk of recurrence), surgical resection with preservation of the artery, or patch angioplasty.
Diagnosis
Popliteal artery entrapment syndrome.
Pearls
Atherosclerotic and embolic disease encompasses the majority of cases of popliteal artery occlusion.
Traumatic occlusion of the popliteal artery is evaluated with angiography and treated surgically.
Popliteal artery aneurysms are often bilateral and are associated with abdominal aortic aneurysms.
Popliteal artery entrapment syndrome is due to abnormal relationship between the artery and musculature.
Suggested Reading
Wright LB, Matchett WJ, Cruz CP, et al. Popliteal artery disease: diagnosis and treatment. Radiographics. 2004; 24(2):467–479Case 29
Clinical History
A 58-year-old man with right common femoral artery access at the end of a liver chemoembolization (Fig. 29‑1 ).

Key Finding
Options for common femoral arteriotomy closure.
Top 3 Methods
Active closure devices. These devices actively close the arteriotomy, with the goal of improved time to ambulation, less patient discomfort, the ability to apply in the setting of anticoagulation, and less time to achieve hemostasis for the operator. However, there is at least theoretical worsening of infection and ischemic complications. Methods of active closure include the following:
Plugs: The collagen plug devices leave an absorbable footplate and knotted plug at the arteriotomy (e.g., AngioSeal, VasoSeal) or a collagen “hydrogel” placed outside the arteriotomy while the vessel lumen is protected by a semicompliant balloon (e.g., Mynx), in order to achieve hemostasis while leaving behind only absorbable material. Other devices with similar absorbable plugs include a polyglycolic acid plug (e.g., ExoSeal), and another that uses porcine small intestine submucosa as an extracellular matrix patch (e.g., FISH).
Clips: Another concept for active closure is the placement of a nitinol implant to clip closed the arteriotomy (e.g., StarClose).
Suture: Most closely following principles of vascular surgical vessel closure, these devices deploy a loop of nonabsorbable suture material through the edges of the arteriotomy and provide a way to approximate the walls and deliver a knot to hold the closure (e.g., Perclose).
Passive closure devices. While not plugging or bringing together the arteriotomy for closure, these devices either provide external compression or aid hemostasis with procoagulant material. Compression devices include a belt placed across the hips, with an inflatable pneumatic bubble deployed just over the arteriotomy site (e.g., FemoStop) or a C-arm clamp placed at the arteriotomy (e.g., ClampEase). They are meant to take the place of the operator and have a high technical success rate. Each of the hemostatic pads (e.g., Chito-Seal, Clo-Sur PAD, Syvek Patch, Neptune Pad, and D-Stat Dry) uses a proprietary procoagulant that speeds time to hemostasis while the operator provides manual compression. These devices require patients to remain at bed rest (similar to manual compression), but free up the operator to continue with additional duties. They do not leave behind any material, and so they are theoretically better than active closure devices in risk of infection or ischemic complications.
Manual compression. This technique remains the “gold standard” in achieving hemostasis. When other devices fail, manual compression is the typical fall back. With manual compression, the catheter or sheath is removed, and firm compression is applied by the operator using the hands. Firm pressure is initially applied (perhaps for the first 10 minutes), followed by lessening degrees (about 20 minutes total for a 5 to 6 F arteriotomy). Additional time may be required for larger accesses or if bleeding persists. Additionally, if anticoagulation is being used, the activated clotting time (ACT) should be decreased to less than 170 seconds prior to attempting manual compression hemostasis. After hemostasis, the patient must lay flat for about 6 hours (~ 1 hour per French size), with the limb maintained straight. Although time consuming, this method of closure has been traditionally touted as the safest.
Diagnosis
Active arteriotomy closure device (AngioSeal).
Pearls
The safety of vascular closure devices cannot be assumed due to “class effect.” For example, the VasoSeal collagen plug has been shown to have increased major complications compared with manual compression, while the AngioSeal has been shown to decrease major complications in published meta-analysis.
Patients at high risk of bleeding due to clinical factors likely benefit from closure devices (active).
Ultrasound-guided access and/or femoral angiography may improve safety associated with vascular closure.
Suggested Reading
Schwartz BG, Burstein S, Economides C, Kloner RA, Shavelle D, Mayeda, G. Review of vascular closure devices. Cath Lab Digest 2011;19(7):10–20Case 30
Clinical History
A 44-year-old woman with blunt trauma and hematuria (Fig. 30‑1 ).

Key Finding
Intraparenchymal renal artery aneurysms.
Top 3 Differential Diagnoses
Vasculitis. Vascular inflammatory conditions are the major cause of intraparenchymal renal aneurysms. The most common condition to affect the small-to-medium–sized renal arteries is polyarteritis nodosa (PAN). This is usually idiopathic, but can be associated with cryoglobulinemia, leukemia, rheumatoid arthritis, Sjogren’s syndrome, and hepatitis B (mnemonic “CLASH”). Patients are usually middle-aged suffering from peripheral neuropathy, intestinal ischemia, and livedo reticularis. Patients with Wegener’s vasculitis present with recurrent sinusitis or epistaxis, mucosal ulcerations, hemoptysis, tracheal stenosis, and eye involvement. Other vasculitides affecting the renal arteries include Takayasu and Churg–Strauss. Treatment involves corticosteroid administration, sometimes with the addition of less proven immunosuppressive agents.
Mycotic/septic emboli. Sources of septic emboli include bacterial endocarditis (often associated with intravenous [IV] drug abuse), infected valves, infected atrial thrombus, or noncardiac sources, and can lead to multiple renal pseudoaneurysms. Typical organisms are gram-positive bacteria (Streptococcus and Staphylococcus subspecies). Treatment is supportive, and antibiotic therapy is directed toward the offending organism while accounting for susceptibility.
Trauma. Aneurysms are usually solitary with a history of recent blunt or penetrating trauma. Iatrogenic injury has led to an increase in this condition, with rupture being the most dreaded complication. Coils, stent grafts, open surgical repair, or nephrectomy are effective treatment options depending on the anatomical location of the aneurysm and clinical scenario.
Additional Diagnostic Considerations
Ehlers–Danlos: Ehlers–Danlos is a connective tissue disorder characterized by abnormal collagen synthesis, which results in skin hyperelasticity, joint hyperextensibility, and vascular fragility. Aneurysms can be seen in multiple vascular beds with mass effect or bleeding. Early diagnosis results in minimizing invasive vascular procedures, which can be catastrophic (especially in type IV Ehlers–Danlos).
Speed kidney: Chronic oral amphetamine use (50 mg daily for 22 years to 200 mg daily for 2 years) may be an independent risk factor for multiple renal artery aneurysms. These patients have no other risk factors but present with multiple visceral artery aneurysms.
Diagnosis
Multiple intraparenchymal renal artery aneurysms (due to trauma).
Pearls
PAN classically results in multiple aneurysms involving small- and medium-sized renal arteries.
Mycotic/septic emboli may result from cardiac or noncardiac sources; multiple pseudoaneurysms are seen.
Blunt or penetrating trauma, along with iatrogenia, may cause renal artery aneurysms.
Chronic amphetamine usage predisposes patients to multiple renal artery aneurysms (speed kidney).
Suggested Readings
Bloch R, Hoffer E, Borsa J, Fontaine A. Ehlers-Danlos syndrome mimicking mesenteric vasculitis: therapy, then diagnosis. J Vasc Interv Radiol. 2001; 12(4):527–529 Nosher JL, Chung J, Brevetti LS, Graham AM, Siegel RL. Visceral and renal artery aneurysms: a pictorial essay on endovascular therapy. Radiographics. 2006; 26(6):1687-1704, quiz 1687 Roane DW, Griger DR. An approach to diagnosis and initial management of systemic vasculitis. Am Fam Physician. 1999; 60(5):1421–1430 Welling TH, Williams DM, Stanley JC. Excessive oral amphetamine use as a possible cause of renal and splanchnic arterial aneurysms: a report of two cases. J Vasc Surg. 1998; 28(4):727–731Case 31
Clinical History
A 33-year-old woman with vague, postprandial abdominal pain and weight loss (Fig. 31‑1 ).

Key Finding
Celiac artery stenosis/occlusion.
Top 3 Differential Diagnoses
Atherosclerosis. Atherosclerosis is the most common cause of narrowing of the mesenteric vessel origins. Patients with atherosclerosis typically present at an older age, have irregularity of multiple vessels, and commonly have associated peripheral vascular disease and/or abdominal aortic aneurysm. Individuals with inadequate collateral circulation will usually present with intestinal ischemia (postprandial pain) and “food ear,” leading to weight loss. Management usually involves angioplasty or stent placement over surgery.
Thromboembolism. Thromboembolic disease is usually from a cardiac (e.g., atrial fibrillation) or proximal aortic source. The celiac axis is rarely affected in comparison to the superior mesenteric artery, cerebral circulation, or peripheral vessels. When intra-abdominal vessels are involved, patients usually present with acute mesenteric ischemia. Affected vessels may have a filling defect or be occluded on angiography. Treatment options center on catheter-directed thrombolysis with or without open surgery in cases of bowel infraction.
Vasculitis. Patients with vasculitis present with a multitude of clinical features, to include constitutional symptoms. Vasculitis may be a primary syndrome (e.g., Takayasu) or secondary to underlying infection or malignancy. The main treatment is corticosteroid administration for primary vasculitides. Catheter-directed treatments may be offered if there is significant end-organ involvement in the fibrotic phase due to stenosis.
Additional Diagnostic Considerations
Median arcuate ligament syndrome: Median arcuate ligament syndrome typically affects thin women between 20 and 40 years of age who present with vague postprandial abdominal pain. A low insertion of the median arcuate ligament (a fibrous arch that unites the diaphragmatic crura on either side of the aortic hiatus) causes narrowing of the celiac axis. Angiography reveals an indentation along the superior aspect of the celiac artery, which is worse during expiration. Surgical decompression is the treatment of choice; however, there is controversy regarding treatment, since many patients have this abnormality without symptoms.
Fibromuscular dysplasia (FMD): FMD most commonly occurs in younger, female patients. Imaging findings include a characteristic beaded appearance secondary to alternating regions of stenosis and dilation. Angioplasty is the primary treatment option in symptomatic patients.
Radiation: Radiation changes usually lead to multiple, smooth, long-segment regions of vessel narrowing. Endoluminal treatments (e.g., stent placement) are the norm for symptomatic lesions.
Diagnosis
Median arcuate ligament syndrome.
Pearls
Atherosclerotic and embolic disease constitutes most cases of mesenteric vessel stenosis or occlusion.
Vasculitides (Takayasu’s arteritis) may cause narrowing of aortic branch vessels; treatment is with steroids.
Median arcuate ligament syndrome affects young, thin women who present with postprandial pain.
Suggested Readings
Horton KM, Talamini MA, Fishman EK. Median arcuate ligament syndrome: evaluation with CT angiography. Radiographics. 2005;25:1177–1182Case 32
Clinical History
A 66-year-old man with worsening hypertension, and renal failure while on an angiotensin-converting enzyme (ACE) inhibitor (Fig. 32‑1 ).

Key Finding
Renal artery stenosis.
Top 3 Differential Diagnoses
Atherosclerosis. The most common cause of renal artery stenosis, atherosclerotic plaques usually form at the ostia (first centimeter) of the renal artery due to disease of the aorta. Patients may be asymptomatic or suffer from renovascular hypertension, chronic renal failure, or episodes of flash pulmonary edema. In the setting of symptoms, intervention is usually offered. The mainstay intervention has become percutaneous stent placement. Despite a high technical success rate (> 98%), clinical responses are variable and difficult to predict. For example, in the setting of renovascular hypertension, approximately one-third have improvement in blood pressure, one-third stabilize, and one-third continue to deteriorate. Reasons for failure include contrast-induced nephropathy, progressive nephrosclerosis, diffuse distal renal artery atherosclerotic disease, and atheroembolism. The theory of atheroembolism has led to the development of low-profile systems for interventions and distal embolic protection devices. Embolic protection appears safe to incorporate into the procedure but is still not universally applied. Lastly, the decision to treat stenosis is somewhat controversial. Most current studies, although not definitive, show no benefit to stent placement over maximal medical management (especially with the use of statins for plaque stabilization or even regression). Although some espouse early treatment despite lack of symptoms, most currently consider invasive intervention only in the setting of the symptoms mentioned previously.
Fibromuscular dysplasia (FMD). Although relatively uncommon, FMD is the leading cause of curable hypertension and most often occurs in younger females. The most common form is that of medial fibroplasia (about 90% of all FMD), which has the typical angiographic appearance of a “string of beads” with alternating regions of stenosis and dilation. The other subtypes may present with stenosis similar to atherosclerosis. Patients usually present with renovascular hypertension but are younger than is typical for atherosclerosis. Angioplasty is highly effective with 80% improvement in hypertension at 1 month and 93% at 2 years. Angioplasty can be repeated as needed with similar clinical results. Other medium-sized vessels may also be affected (e.g., internal carotid artery).
Dissection. Dissections often begin in the aorta (ascending or descending) and can affect the perfusion and origin caliber of the renal arteries. Iatrogenic dissection of the renal artery can occur with diagnostic angiography or endoluminal interventions. Treatment is based on the morphology of the dissection and clinical symptoms with operative therapy, fenestration, or stent placement all utilized.
Diagnosis
Symptomatic atherosclerotic stenosis of the right mid renal artery treated with a stent.
Pearls
Atherosclerosis is the most common cause of renal artery stenosis and typically involves the renal ostia.
Despite technical success of renal artery stents for atherosclerotic lesions, clinical response is variable.
FMD most often occurs in young females; lesions are treated with angioplasty, which is highly effective.
Treatment of renal artery dissections is based on the morphology of the dissection and clinical symptoms.
Suggested Readings
Kim HJ, Do YS, Shin SW, et al. Percutaneous transluminal angioplasty of renal artery fibromuscular dysplasia: mid-term results. Korean J Radiol. 2008; 9(1):38–44 Misra S, Gomes MT, Mathew V, et al. Embolic protection devices in patients with renal artery stenosis with chronic renal insufficiency: a clinical study. J Vasc Interv Radiol. 2008; 19(11):1639–1645 van Bockel JH, Weibull H. Fibrodysplastic disease of the renal arteries. Eur J Vasc Surg. 1994; 8(6):655–657Case 33
Clinical History
A 50-year-old woman with hematuria (Fig. 33‑1 ).

Key Finding
Hypervascular renal mass.
Top 3 Differential Diagnoses
Renal cell carcinoma (RCC). In adults, RCC comprises over 80% of all malignant renal masses. It is more common in older males and may be multiple when associated with von Hippel–Lindau disease. Angiographically, RCC most often has prominent neovascularity (94%) and shunting with or without venous invasion. On CT, RCC presents as a hypervascular mass. When large, they may become centrally necrotic. The central hypodensity in these instances should not be confused with fat, as is seen in benign angiomyolipomas (AMLs). The typical indication for angiographic intervention is usually 24-hour preoperative embolization of the tumor or kidney to decrease intraoperative hemorrhage. Devascularization with smaller particles for peripheral embolization is preferred, provided no arteriovenous (AV) shunts are present. Otherwise, alcohol ablation with balloon occlusion catheter or coils may be used. Smaller RCC can be treated with nephron-sparing surgery or radiofrequency (RF)/cryoablation. RCC commonly produces hypervascular hepatic metastases and lytic bone lesions.
Angiomyolipoma. AMLs are benign hamartomatous lesions that contain fat, smooth muscle and blood vessels. The typical patient is a female between 30 and 60 years. Multiple bilateral lesions are found in up to 80% of patients with tuberous sclerosis. Large lesions (> 4 cm) are prone to acute spontaneous hemorrhage. On CT, a fat-containing mass originating from the kidney is virtually pathognomonic. Angiography demonstrates neovascularity and bizarre aneurysms without vascular shunting. Embolization with permanent particles or alcohol is an excellent alternative to resection.
Oncocytoma. Oncocytomas are solid benign renal neoplasms composed of oncocytes. They are usually asymptomatic, but may present with hematuria, flank pain, and/or an abdominal mass, mimicking RCC. The lesions are typically large (> 5 cm) at presentation and have a pseudocapsule. On CT, the lesions are hypervascular, and up to one-third will have a central stellate scar. The classic angiographic finding is a “spokewheel” arrangement of tumor vascularity. Although the diagnosis may be suggested by imaging findings, there is significant overlap with the findings of RCC.
Diagnosis
Renal cell carcinoma.
Pearls
RCC is the most common renal tumor in adults; it is hypervascular with neovascularity and shunting.
Large AMLs (> 4 cm) are prone to hemorrhage secondary to neovascularity and bizarre aneurysms.
Oncocytomas are solid benign renal neoplasms with a central scar and “spokewheel” pattern of vascularity.
Suggested Readings
Davidson AJ, Hartman DS, Choyke PL, Wagner BJ. Radiologic assessment of renal masses: implications for patient care. Radiology 1997;202(2):297–305 Israel GM, Bosniak MA. How I do it: evaluating renal masses. Radiology. 2005; 236(2):441–450 Kaufman JA, Lee MJ. Vascular and Interventional Radiology: The Requisites. Philadelphia, PA: Elsevier; 2004;6–11:337–346Case 34
Clinical History
A 71-year-old woman with ipsilateral transient ischemic attacks (Fig. 34‑1 ).

Key Finding
Carotid artery stenosis.
Top 3 Differential Diagnoses
Atherosclerosis. Atherosclerosis is the most common cause of carotid artery stenosis. It most often occurs in older individuals and is more advanced with smoking history, hypertension, hyperlipidemia, and diabetes. The carotid bifurcation and proximal internal carotid artery are the most common sites involved, although atherosclerosis may involve any segment, including intracranially. Doppler ultrasound is the initial screening study. Typical findings on ultrasound include visualization of plaque with vessel stenosis, elevated velocities, and turbulent flow with aliasing. MRA and CTA are used as confirmatory studies. Carotid endarterectomy (CEA) remains the treatment of choice in low-risk surgical candidates. Current guidelines recommend CEA for symptomatic lesions with greater than 50% stenosis (data from NASCET trial) or asymptomatic lesions with greater than 60% stenosis (data from ACAS trial). Although many recent trials have shown a benefit of endovascular carotid stent placement with distal embolic protection devices in many patient populations, current guidelines recommend this treatment only for high-risk surgical patients with symptomatic lesions of greater than or equal to 70% stenosis. High-risk surgical patients are defined as follows: clinically significant cardiac disease, severe pulmonary disease (if general anesthesia will be used), recurrent stenosis after prior CEA, prior neck surgery or irradiation, contralateral recurrent laryngeal nerve paralysis, contralateral carotid artery occlusion, and surgically inaccessible lesions (high or low lesions).
Fibromuscular dysplasia (FMD). FMD is a dysplastic vascular disease which primarily affects young women. The renal arteries are most commonly involved, followed by the carotid arteries. The disease results in vascular stenosis and resultant hypertension (renal artery) or stroke (internal carotid artery). Patients are at increased risk of aneurysms and dissections. The medial fibroplasia variant is the most common subtype and presents with the classic “string of beads” appearance due to alternating regions of stenosis and dilation. Symptomatic lesions are treated with angioplasty.
Dissection. Carotid artery dissection may be due to hypertension, trauma, iatrogenia, or even FMD. Most nontraumatic dissections can be managed medically with some form of anticoagulation and follow-up imaging (usually CTA) to ensure stability or resolution. Symptomatic lesions or lesions which progress despite medical management often require intervention, to include stent placement.
Additional Diagnostic Consideration
Iatrogenic: The most common iatrogenic causes of carotid stenosis include clamp injury during surgical procedures or vasculitis secondary to radiation therapy for head and neck cancer. Treatment depends on symptoms and morphology of the lesions with endovascular therapy favored over surgical intervention.
Diagnosis
Symptomatic atherosclerotic stenosis of the left internal carotid artery treated with a stent.
Pearls
Atherosclerosis is the most common cause of carotid artery stenosis; it typically occurs in older individuals.
Carotid stents are indicated for symptomatic lesions of greater than or equal to 70% stenosis in high-risk surgical patients.
FMD affects primarily young females; angioplasty is the primary treatment option for symptomatic lesions.
Most dissections are due to hypertension or trauma; treatment depends on morphology and symptoms.
Suggested Readings
Barnett HJM, Taylor DW, Haynes RB, et al; North American Symptomatic Carotid Endarterectomy Trial Collaborators. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med. 1991; 325(7):445–453 Executive Committee for the Asymptomatic Carotid Atherosclerosis Study (ACAS). Endarterectomy for asymptomatic carotid artery stenosis. JAMA. 1995; 273:1421–1428 Gurm HS, Yadav JS, Fayad P, et al; SAPPHIRE Investigators. Long-term results of carotid stenting versus endarterectomy in high-risk patients. N Engl J Med. 2008; 358(15):1572–1579 Roffi M, Yadav JS. Carotid stenting. Circulation 2006; 114(1):e1-e4Case 35
Clinical History
A 38-year-old woman with bilateral hand pain and intermittent pallor (Fig. 35‑1 ).

Key Finding
Digital artery occlusions.
Top 3 Differential Diagnoses
Raynaud’s syndrome. Raynaud’s syndrome is a vasospastic disorder prompted by cold temperatures. It represents the most common cause of upper extremity ischemia. Imaging findings include stenosis or occlusion of digital artery branches that are relieved by warming of the affected extremity or administration of a vasodilator. Although Raynaud’s syndrome is associated with scleroderma, other connective tissue disorders can produce similar clinical symptoms and radiographic findings.
Vasculitis. Vasculitis may result in upper extremity ischemia and has a variety of etiologies, to include systemic lupus erythematosus, connective tissue disorders, and Buerger’s disease. Imaging findings typically include stenosis or occlusion of arteries within the hand or wrist. Systemic steroids provide relief of acute episodes. Buerger’s disease (thromboangiitis obliterans) occurs predominantly in young male smokers. It affects the lower extremities alone in 20%, upper and lower in 75%, and upper alone in 5%. In chronic disease, classic “corkscrew collaterals” develop. Symptoms are improved with smoking cessation.
Embolic disease. Embolic disease may emanate from either the heart or within the vascular supply to the affected upper extremity. Cardiac emboli constitute the most common cause of acute upper extremity ischemia. Imaging findings include arterial occlusions most commonly within the small vessels of the hand(s). A cardiac source usually involves both upper extremities, while a source from a more proximal artery results in unilateral disease. The entire extremity should be evaluated to find lesions amenable to percutaneous interventions. Thrombolysis may be beneficial in certain circumstances.
Additional Diagnostic Considerations
Thoracic outlet syndrome: Thoracic outlet syndrome results from chronic compression of the upper extremity neurovascular structures secondary to abnormal insertion of scalene musculature or the presence of a cervical rib. Imaging findings include stenosis or occlusion of the subclavian artery, arterial injury (aneurysm or pseudoaneurysm), and distal emboli. If the angiogram in the neutral position is normal, the angiogram should be repeated with the affected extremity abducted and externally rotated. Percutaneous treatment may be beneficial but the definitive treatment is surgical correction of the compressive lesion.
Trauma: Frostbite, burns, blunt/penetrating injury or repetitive injury can involve the hands. Frostbite: It results in occlusion of the digital arteries with sparing of the thumbs secondary to clenched fists with the digits protecting the thumbs. Other patterns of vascular injury follow the distribution of the trauma.
Atherosclerosis: Atherosclerotic disease constitutes the most common cause of chronic upper extremity ischemia. Although any artery may be involved, the digital arteries are affected in patients with chronic hypertension, diabetes, and/or renal failure. Imaging findings include stenosis or occlusion of the arteries of the wrist, hand, or digits. The vascular supply of the entire extremity should be interrogated to find lesions amenable to percutaneous interventions.
Diagnosis
Vasculitis.
Pearls
Raynaud’s syndrome is vasospasm due to cold temperatures; it is relieved with warming or vasodilators.
Many vasculitides may cause digital artery occlusion; Buerger’s disease results in “corkscrew collaterals.”
Embolic disease from a proximal source (cardiac or thoracic outlet) may cause digital artery occlusion.
Suggested Reading
Kaufman JA, Lee MJ. Vascular and Interventional Radiology: The Requisites. Philadelphia, PA: Elsevier Inc.; 2004Case 36
Clinical History
A 50-year-old woman with fever, malaise, and right arm pain (Fig. 36‑1 ).

Key Finding
Vasculitis.
Top 3 Classification (Most Common)
Large vessels.
Giant cell arteritis: Most common vasculitis of adulthood (age ~ 50); females are more affected than males. Segmental granulomatous inflammation (with giant cells). Causes unilateral headache, ipsilateral visual loss, polymyalgia rheumatic, fever, malaise. Diagnosed by biopsy of temporal artery. Treated with corticosteroids with good prognosis (subsides in 6–12 months); blindness if left untreated.
Takayasu’s arteritis (“pulseless disease”): Age less than 40; Asians, females are more affected than males. Granulomatous inflammation with intimal fibrosis and giant cell granulomas. Causes symptoms associated with decreased blood flow to upper arms and head. Treated with steroids with good prognosis unless present with stroke or aortic dissection.
Medium Vessels.
Kawasaki’s disease (mucocutaneous lymph node syndrome): Infants/young children. Transmural necrotizing arteritis induced by viral or bacterial superantigens. Causes coronary artery changes (70%; aneurysm), skin rash, adenopathy, fever, malaise, conjunctival and oral erosions, and erythema. Treated with aspirin and intravenous (IV) gamma globulin. Usually, self-limiting except must beware of cardiac involvement (acute myocardial infarction or rupture of aneurysm).
Polyarteritis nodosa: Young adults, males more commonly affected than females, 30% with hepatitis B antibody. Segmental transmural necrotizing arteritis (multiple stages may coexist). Causes nephritic syndrome, renal failure, gastrointestinal (GI) bleeding, diffuse muscular pains, painful skin subcutaneous nodules, fever, malaise, weight loss. Treated with immunosuppression. Fatal if left untreated.
Small Vessels.
Granulomatosis with polyangiitis (formerly Wegener’s granulomatosis): Adults (any); M ≥ F. Granulomatous vasculitis. Causes necrotizing lung granulomas, recurrent pneumonia, necrotizing glomerulonephritis, nephritic syndrome, chronic sinusitis, nasopharyngeal ulcers. Treated with steroids; fatal if untreated.
Churg–Strauss syndrome (eosinophilic granulomatosis and polyangiitis): Age about 50; M ≥ F. Very rare. Necrotizing granulomatous vasculitis with eosinophils. Leads to asthma, allergic paranasal sinusitis, eosinophilia, and transient pulmonary infiltrates and purpuric rash.
Microscopic polyangiitis: Age about 50; M ≥ F, White > Black. Segmental necrotizing vasculitis thought to be caused by hypersensitivity reaction to agent (e.g., drug [aspirin, penicillin, thiazide diuretic] or microbes). Causes purpuric rash of lower extremities, glomerulonephritis, sinusitis, pneumonitis, hemoptysis, mucosal bleeding (GI). Treatment is removal of offending agent. Prognosis is good except when renal or respiratory failure.
Henoch-Schönlein purpura: Most common vasculitis of children; immunoglobulin A (IgA)-associated vasculitis (IgA-complement immune deposits). Involves skin, GI tract, kidneys, and joints. This condition is self-limited.
Not vessel size specific: Behçet’s, Cogan’s, relapsing polychondritis.
Diagnosis
Giant cell arteritis (also known as temporal arteritis).
Pearls
In patients with vasculitis-like symptoms, must initially rule out infection (e.g., syphilis, HIV), neoplasm, inflammatory bowel disease, or drug effects (all usually with small vessel changes).
Patients with unexplained systemic illness with multiple system involvement (e.g., upper airway, renal, pulmonary, dermatologic, neurologic) should be tested for vasculitis.
Suggested Reading
Systemic Vasculitis. https://arupconsult.com/content/vasculitis. Accessed May 17, 2016Case 37
Clinical History
Patients with arterial occlusive disease.
Key Finding
Patterns of arterial occlusive disease (Fig. 37‑1 ).

Top 3 Differential Patterns
Smoker. Those who smoke are prone to arterial occlusive disease. The pattern is often described as proximal, involving the aortoiliac and proximal femoral arteries. Given this pattern, when smokers present with symptoms associated with arterial occlusive disease, they most often suffer from intermittent claudication.
Diabetes and/or chronic renal failure. Arterial occlusive disease, in diabetic and renal failure patients, tends to be distal in the extremities, generally considered “femoral popliteal and tibial” occlusive disease. The named vessels in the calf, most notably the peroneal and posterior tibial arteries, are more often occluded in those with diabetes, relative to nondiabetic smokers. However, occlusion of the pedal arches seems similar between these two groups. Given this distribution, and other factors, diabetic patients tend to present with tissue loss.
Nonatherosclerotic/isolated. Isolated arterial occlusive disease is often noted in those with nonatherosclerotic etiologies. Nonatherosclerotic peripheral arterial disease is a heterogeneous group of uncommon conditions. Conditions include popliteal artery entrapment, cystic adventitial disease, midaortic syndrome, vasculitis, fibromuscular dysplasia, Buerger’s disease, and endofibrosis. Beware of nonatherosclerotic peripheral arterial occlusive disease in patients younger than 50 years, who lack classic risk factors (e.g., smoking, diabetes), or have a sudden onset/waxing–waning of symptoms.
Diagnosis
Comparison of diabetic versus smoking patterns of peripheral arterial occlusive disease.
Pearls
Of those presenting with symptomatic infrainguinal arterial disease, diabetic patients are significantly more likely to present with gangrene or ulcer compared to nondiabetic smokers (about 75 vs. 40%, respectively).
Over 90% of those presenting with intermittent claudication are smokers.
Presence of atherosclerotic peripheral arterial vascular occlusive disease is associated with an increased risk of myocardial infarction, stroke, and all-cause mortality.
Nonatherosclerotic disease is usually isolated, and the diagnosis may be specific to the region of stenosis or occlusion (e.g., popliteal artery entrapment will have normal vessels throughout, except at the knee).
Suggested Readings
Menzoian JO, LaMorte WW, Paniszyn CC, et al. Symptomatology and anatomic patterns of peripheral vascular disease: differing impact of smoking and diabetes. Ann Vasc Surg. 1989; 3(3):224–228 Weinberg I, Jaff MR. Nonatherosclerotic arterial disorders of the lower extremities. Circulation 2012; 126(2):213–222Case 38
Clinical History
A 50-year-old woman with heme-positive stools (Fig. 38‑1 ).

Key Finding
Hypervascular subepithelial small bowel mass.
Top 3 Differential Diagnoses
Neuroendocrine tumors. These tumors originate from enteroendocrine cells located throughout the gastrointestinal (GI) tract (in decreasing order of occurrence: appendix, small bowel, rectum, colon, and stomach). Some of these tumors release vasoactive amines, such as serotonin by carcinoid tumors. When metastatic to the liver, secreted serotonin bypasses metabolism in the liver and can result in carcinoid syndrome (occurs in < 10% of carcinoid patients; consists of cutaneous flushing and diarrhea). The World Health Organization has classified neuroendocrine tumors into three classifications: well-differentiated (e.g., typical carcinoid, low-grade malignancy), well-differentiated carcinomas (e.g. atypical carcinoid), and poorly differentiated, high-grade malignancies. Many small bowel neuroendocrine tumors behave aggressively, undergoing transmural extension and metastasis to lymph nodes. Metastases to the mesentery form a mass (often larger than the primary) with a sunburst pattern on CT (e.g., desmoplastic reaction).
Mesenchymal tumors. Tumors of the mesenchyme are from cells that develop into connective tissue, or the lymphatic or circulatory system.
Gastrointestinal stromal tumors (GISTs): Although more commonly from the stomach (60%), about 30% of all GISTs originate in the small bowel. They are usually solitary. Staging is based on size, dissemination, and mitotic rate. By CT, features of aggressiveness include irregular lobulated margins, ulceration, central necrosis, heterogeneous enhancement, and presence of metastasis.
Vascular tumors: These are rare entities, and include glomus tumors, hemangiomas, Kaposi’s sarcoma, and angiosarcoma.
Nerve sheath tumors: Differential includes GI schwannoma and the even rarer paragangliomas.
Hypervascular metastasis. Spread of malignancy to the small bowel occurs via direct extension, peritoneal implantation to the serosa, or hematologic spread. These are almost always multifocal. Most common primaries to spread to the small bowel are melanoma and breast cancer. Less common primaries include renal cell carcinoma, choriocarcinoma, neuroendocrine tumors, and mesenchymal tumors.
Additional Diagnostic Considerations
Heterotopic tissues: These are tissues that are separate from the main organ, lacking vascular or ductal connections. Heterotopic pancreas occurs in 1 to 15% of the population, usually along the upper GI tract (e.g., greater curvature of the stomach), but can occur on the small bowel. It is usually asymptomatic, but may present with pain, bleeding, or obstruction. Heterotopic gastric mucosa can be found along the small bowel, sometimes associated with duplication cysts or a Meckel diverticulum. Although truly mucosal, they are sometimes mistaken for the aforementioned subepithelial lesions.
Varices: These are sometimes mass-like and polypoid, and may be difficult to differentiate from other masses by endoscopy (if deep). CT can be helpful in determining extent of varices and possible etiologies.
Diagnosis
Small bowel carcinoid tumor.
Pearls
CT is important in evaluation of these tumors as it can demonstrate local invasion, and distant metastasis. This information is complementary to that found by histological examination.
With carcinoid, up to 30% are multicentric. Enteroclysis or CT may be helpful to find additional foci.
Primary small bowel adenocarcinoma and lymphoma enhance but are usually not hypervascular.
Suggested Reading
Lee NK, Kim S, Kim GH, et al. Hypervascular subepithelial gastrointestinal masses: CT-pathologic correlation. Radiographics. 2010;30(7):1915–1934Case 39
Clinical History
A 45-year-old woman with incidental liver mass (Fig. 39‑1 ).

Key Finding
Hypervascular liver mass in the noncirrhotic liver.
Top 3 Differential Diagnosis (Benign)
Hemangioma. These are circumscribed, blood-filled spaces lined by endothelium and thin stroma. Hemangiomas are the most common hepatic tumor. Imaging with contrast classically (about 75% of cases) shows peripheral, discontinuous enhancement that is equal to the blood pool on all phases, and slowly fills the entire mass. Other enhancement patterns include the flash fill hemangioma (immediate, uniform enhancement in a small [< 1.5 cm] mass), and a giant hemangioma (> 5 cm) that has persistent hypoenhancement centrally.
Focal nodular hyperplasia (FNH). The second most common benign liver tumor, FNH is a hyperplastic response of the hepatic parenchyma to an arterial malformation. The mass is made of hepatocytes, Kupffer’s cells (many more than adenomas), and small bile ducts surrounding a central scar. The scar shows delayed enhancement postcontrast administration as it is composed of malformed vascular structures. They are usually asymptomatic, rarely causing pain from capsular stretch, mass effect, or blood flow alterations.
Adenoma. Hepatic adenomas are most common in women taking oral contraceptives. They are usually solitary; when multiple, they are associated with glycogen storage disease or anabolic steroids. They may contain fat. Symptoms are a bit more common than with FNH, and there is a risk of rupture and bleeding when larger than 5 cm. Lastly, there is a rare risk of malignant transformation.
Additional Considerations (Malignant)
Hepatocellular carcinoma (HCC): Only 20% of all HCC occur in the noncirrhotic liver. A subtype called fibrolamellar hepatocellular carcinoma is important to recognize as it can be confused with FNH. Both are found in younger patients and have a central scar. However, the scar in fibrolamellar HCC is low intensity on T2-weighted images (and on all other sequences) and does not enhance. Fibrolamellar HCCs lack Kupffer’s cells (they do not take up Tc99m sulfur colloid).
Solitary hepatic metastasis: Metastases are common to the liver. Unlike typical hypovascular masses, which are conspicuous on portal venous phased imaging, hypervascular metastatic disease is best seen on arterial phase imaging. Hypervascular metastases originate from melanoma, renal cell carcinoma, choriocarcinoma, thyroid cancer, breast cancer, lung cancer, sarcomas, and neuroendocrine tumors (e.g., islet cell tumors, carcinoid, and pheochromocytoma).
Diagnosis
Hypervascular liver mass on CT (ultimately diagnosed as focal nodular hyperplasia).
Pearls
In symptomatic giant hemangiomas (> 5 cm), particle transarterial embolization may be used to improve pain, halt growth, reduce mass effect, correct coagulation disturbances, and reverse inflammatory syndromes. Others consider giant hemangiomas as greater than 10 cm, and advocate embolization to prevent rupture and bleeding.
Transient hepatic attenuation differences (THAD) or transient hepatic intensity differences (THID) are bright on arterial phase, but isodense/isointense under all other imaging conditions.
Hemangiomas are more T2 bright than most metastasis. To differentiate between the two, delayed contrast phases show washout for metastasis, but retain contrast in the setting of hemangiomas.
Suggested Reading
Silva AC, Evans JM, McCullough AE, Jatoi MA, Vargas HE, Hara AK. MR imaging of hypervascular liver masses: a review of current techniques. Radiographics. 2009;29(2):385–402Case 40
Clinical History
A 54-year-old woman with chronic dyspnea (Fig. 40‑1 ).

Key Finding
Pulmonary artery enlargement.
Top 3 Differential Diagnoses
Pulmonary hypertension. Nonfocal enlargement of the central pulmonary vasculature is usually due to pulmonary hypertension. Pulmonary hypertension is defined as a mean pulmonary artery pressure greater than or equal to 25 mm Hg. CT and MR are excellent tools to determine pulmonary artery enlargement, with 29 mm for men, and 27 mm for women (the 90th percentile cutoff values), generally representing the top size for normal vessels. Enlargement of the main pulmonary artery should elicit investigation. Etiologies of pulmonary artery hypertension can be broken down into precapillary (e.g., thromboembolic disease), capillary/combined (e.g., pulmonary fibrosis), and postcapillary (e.g., left heart failure) processes.
Aneurysm/pseudoaneurysm. Focal dilation of the pulmonary artery is most often due to vasculitis (Behçet’s or Hughes–Stovin), infection (e.g., tuberculosis, pyogenic bacteria, or fungi), neoplasm, iatrogenia (e.g., malpositioned Swan–Ganz catheter), trauma, or rare connective tissue abnormalities (e.g., Marfan, Ehlers–Danlos). They are best evaluated by contrast-enhanced chest CT or MR. As they are rare, criteria for intervention may vary, but most advocate for early treatment given the high morbidity and mortality associated with rupture.
Pulmonary arteriovenous malformation (pAVM). A congenital defect in the terminal capillary loop leads to formation of thin-walled vascular sacs and formation of pAVM. The connection leads to a high-flow, low-resistance fistula between pulmonary artery and vein. Most are asymptomatic but can lead to dyspnea (due to right-to-left shunt), and paradoxical emboli, usually presenting in the first three decades of life. There is a strong association between pAVM and hereditary hemorrhagic telangiectasia (HHT), with 33% of those with solitary pAVM having HHT, and over 50% with multiple pAVM having HHT. Classic teaching has been to treat, most often by coil embolization, if the feeding vessel is 3 mm or greater. However, paradoxical emboli have been noted in those with feeding vessels less than 3 mm. Thus, in adult patients, many HHT centers are treating sub-3 mm pAVM. There are no such sub-3 mm guidelines in the pediatric population, except that in the setting of symptomatic patients, where most agree treating small feeding vessel pAVM is warranted.
Diagnosis
Pulmonary hypertension (sequelae of chronic pulmonary emboli).
Pearls
Pulmonary artery diameter of 29 mm or greater has a 97% positive predictive value (sensitivity 87%, specificity 89%) for the presence of pulmonary artery hypertension.
Incidence of rupture and bleed from Swan–Ganz catheters is about 0.2% of patients catheterized.
Presence of pAVM should lead to screening for HHT.
Suggested Readings
Nguyen ET, Silva CI, Seely JM, Chong S, Lee KS, Müller NL. Pulmonary artery aneurysms and pseudoaneurysms in adults: findings at CT and radiography. AJR Am J Roentgenol 2007;188(2):W126–W134 Peña E, Dennie C, Veinot J, Muñiz SH. Pulmonary hypertension: how the radiologist can help. Radiographics 2012;32(1):9–32 Trerotola SO, Pyeritz RE. PAVM embolization: an update. AJR Am J Roentgenol 2010;195(4):837–845Case 41
Clinical History
A 68-year-old man with facial swelling (Fig. 41‑1 ).

Key Finding
Central venous occlusions.
Top 3 Differential Diagnoses
Malignancy. Lung malignancy, both small and non-small cell, is the most common tumor leading to superior vena cava (SVC) obstruction. Obstruction may be due to compression or direct invasion. Lymphoma can also compress the vessel and is the second most common tumor. Other mediastinal tumors leading to obstruction include malignant thymoma, germ cell tumors, mesothelioma, or nodal metastasis. Very rarely is the vena cava primarily affected by malignancy, such as in angiosarcoma or leiomyosarcoma.
Prior instrumentation. The use of medical devices that are risk factors for SVC occlusion has increased. Devices known to cause stenosis or thrombosis of the SVC include larger-bore catheters (e.g., hemodialysis catheters), and cardiac devices (e.g., pacemakers).
Fibrosing mediastinitis. Although histologically benign, this proliferation of collagen and fibrosis within mediastinal tissues can lead to SVC occlusive disease. Occlusion is typically extrinsic as there is no direct invasion of the cava wall. Classically described as idiopathic, it may be caused by an abnormal immunologic response to Histoplasma species or tuberculosis. It may also be related to retroperitoneal fibrosis. On CT, fibrosing mediastinitis appears as an ill-defined, soft tissue mass, particularly in the middle mediastinum, obliterating normal fat planes.
Additional Diagnostic Consideration
Radiation therapy: Radiation-induced fibrosis can also cause extrinsic compressive occlusion to the SVC. A distinctly demarcated distribution in the shape of a radiation field can help suggest the diagnosis. However, history is the key.
Diagnosis
Lung cancer leading to bilateral brachiocephalic-SVC occlusion.
Pearls
Malignancy is responsible for about two-thirds of patients presenting with SVC occlusion. Iatrogenic causes are becoming more common.
Causes of occlusion can also be classified into mass effect, intrinsic stenosis, or thrombosis.
Suggested Reading
Sheth S, Ebert MD, Fishman EK. Superior vena cava obstruction evaluation with MDCT. AJR Am J Roentgenol 2010;194(4):W336–W346Case 42
Clinical History
A 59-year-old man with new-onset liver failure (Fig. 42‑1 ).

Key Finding
Transvenous evaluation techniques in cirrhosis.
Top 3 Techniques
Hepatic venous pressures. The most suitable tool to assess for portal hypertension is catheter-directed pressure measurements. In this procedure, a catheter is placed into a hepatic vein, usually from a transjugular approach, and pressure measurements are taken with the catheter wedged (or with an occlusion balloon) and free floating in the vein. The wedge pressure estimates the portal venous pressure. The wedge pressure, minus the free hepatic venous pressure, is the hepatic venous pressure gradient (HVPG), and is an estimate of the portosystemic gradient (PSG). The normal PSG is 1 to 4 mm Hg; greater than or equal to 5 mm Hg is considered portal hypertension. Nevertheless, “clinically significant portal hypertension” is defined as that above the threshold value of 12 mm Hg and is where symptoms can occur (e.g., variceal bleeding or ascites). Interestingly, however, many patients above this threshold do not have symptoms due to individual differences in collateral circulation. Obtaining a HVPG can provide insight into patient prognosis, is used to direct interventions such as transjugular intrahepatic portosystemic shunt (TIPS), and can help direct management with β-blockers.
Hepatic venography. Venography of the hepatic veins is used to confirm placement of catheters prior to pressure measurements, or before interventions such as TIPS. Additionally, hepatic venography can uncover cirrhosis mimickers, like hepatic venous occlusive disease (e.g., stenosis or webs), that can elevate portal pressures without liver parenchymal disease.
Transjugular liver biopsy. The diagnosis of cirrhosis is made histologically, not clinically or radiographically. Normally, percutaneous core needle biopsy of the liver is adequate for diagnosis. In the setting of uncorrectable bleeding diathesis or severe ascites, a transjugular approach should be utilized to limit complications. Many commercial sets are available. Most sets contain the following: a 7-F, 60-cm long sheath with a curved metal stiffener, a 5-F diagnostic catheter, and an 18-g core biopsy needle (with a 20-mm throw) that is length matched to the sheath and metal stiffener.
Diagnosis
Radiographs showing transvenous techniques in the evaluation of cirrhosis.
Pearls
Direct measurement of PSG post-TIPS can help monitor efficacy, as a reduction of 25 to 50% from the pre-TIPS HVPG has been shown to significantly lower the risk of variceal bleeding.
A preoperative HVPG of greater than 10 mm Hg is a contraindication to hepatic resection for hepatocellular carcinoma (due to the considerably higher risk of periprocedural complications).
A HVPG greater than 20 mm Hg is a predictor of impending failure to successfully manage, either medically or endoscopically, acute variceal bleeding.
Suggested Reading
Merkel C, Montagnese S. Hepatic venous pressure gradient measurement in clinical hepatology. Dig Liver Dis 2011;43(10):762–767Case 43
Clinical History
A 55-year-old man with lower extremity pain (Fig. 43‑1 ).

Key Finding
Peripheral arterial occlusive disease (application of endoluminal technologies).
Top 3 Differential Procedural Options
Percutaneous transluminal angioplasty (PTA). In PTA, vessel blockage is crossed with a wire, and then a balloon catheter is inflated to efface the narrowing. Lesions most amenable to PTA are short in length (< 3 cm), concentric, not completely occlusive, noncalcified, nonostial, and in larger-diameter vessels with good inflow and outflow. Angioplasty is often employed due to cost-effectiveness, low complication rates, and repeatability. Balloons should be sized conservatively, targeting about a 10% greater diameter than the target vessel size, and inflated slowly and gently just to the necessary pressure to efface the lesion.
Percutaneous stent placement. Stents are metallic devices used to open, and maintain open, vessel blockages. There are two basic types: balloon-expandable and self-expanding. Balloon-expandable stents are deployed on a balloon catheter, have good radial force, are able to be precisely placed (no foreshortening; less “jumping”), but are crushable and force the vessel to conform to their straightness. Self-expanding stents are unsheathed from a catheter system, have less radial strength, are slightly less precise in their deployment, have recoil when external forces are applied, and are able to conform to vessel tortuosity. Stents should be utilized in peripheral atherosclerotic occlusive disease in the setting of complete occlusions, suspicion of distal emboli, or when the lesion is located at the ostium. Additionally, stents are used after failed PTA. Failure of PTA is defined as a residual stenosis postangioplasty (of at least 25%), residual pressure gradient (>10 mm Hg systolic), or when flow-limiting dissection is noted. Covered stents or “stent grafts” may be beneficial in maintaining patency and are important tools in dealing with complications (e.g., vessel rupture).
Atherectomy. These devices use rotors or laser energy to damage atheromatous plaques, and then suction to remove resultant debris. They are often utilized in conjunction with other technologies such as angioplasty.
Additional Procedural Consideration
Drug-eluting balloons or stents: Balloons and stents impregnated with antineoplastic (e.g., paclitaxel) or immunosuppressive (e.g., sirolimus or everolimus) agents have been added to the arsenal against peripheral arterial vascular disease. Their goal is to decrease the response to vessel injury associated with interventions and reduce the restenosis rates.
Diagnosis
Single, short-segment occlusive lesion ideal for simple PTA.
Pearls
The use of heparin and nitroglycerin intraprocedurally can help limit complications during endovascular interventions. Prevention of platelet aggregation postprocedurally is also important (e.g., aspirin, glycoprotein IIb/IIIa antagonists, or adenosine diphosphate [ADP] receptor antagonists).
Although there are multiple technologies available to the interventionalist, angioplasty continues to be the mainstay technique for management of most lesions.
Suggested Reading
Tadwalkar RV, Lee MS. The current state of endovascular intervention for peripheral arterial disease. Vascular Disease Management 2015;12(10):E190-E203Case 44
Clinical History
An 83-year-old man with history of atrial fibrillation, presenting with acute leg pain (Fig. 44‑1 ).

Key Finding
Arterial embolus (nonneurologic).
Management of patients depends on the vascular bed affected, presence of collaterals, and the severity and timing of clinical symptoms.
Top 3 Management Options
Catheter-directed pharmacologic thrombolysis. When patients present early, and symptoms are mild, they may be candidates for catheter-directed thrombolysis. The medications used are exogenous plasminogen activators (e.g., tissue plasminogen activator or t-PA). A multiside hole catheter is advanced into the embolus. Pharmaceutical is then continuously dripped into the embolus. Other methods include lacing or blousing the clot, pulse spray, and graded/step-wise infusion (the method of infusion depends on the severity of symptoms and the rapidity required to perform thrombolysis). Continuous infusion using recombinant t-PA proceeds at a rate of 0.5 to 1 mg/h (maximum 40 mg) with an optional bolus of 2 to 5 mg to start. Peripheral administration of a “subtherapeutic dose” of intravenous (IV) heparin, at a rate of about 2 to 500 units/h (target activated partial thromboplastin time [aPTT] of 1.25–1.5 times control), may help limit ongoing thrombus formation. Along with checking coagulation and hematologic parameters, following fibrinogen levels may be helpful (halting infusions or transfusing fresh frozen plasma if levels drop below 100 mg/dL; reducing dose by 50% if levels below 150 mg/dL). Patients are monitored in the intensive care unit (ICU) to check for bleeding complications (e.g., groin hematoma, stroke, gastrointestinal [GI] bleed), manage the many drips, monitor coagulation parameters, and keep the patient comfortable. Patients should return to the angiography suite every 4 to 8 hours to check progress. End points include complete lysis with restoration of flow, lysis to a point of no further benefit, failure to lyse clot, or when a major complication occurs. After thrombolysis, any underlying lesion should be addressed. Note that organized clot in an embolus is less likely to respond than fresh clot.
Catheter-directed mechanical thrombolysis. In the setting of more moderate symptoms, percutaneous mechanical thrombolysis may be beneficial to more quickly reestablish flow. Sometimes, all that is required is placement of a sheath to the embolus, with suction thrombectomy. The first device Food and Drug Administration (FDA) approved for arterial thrombolysis was the AngioJet, which is used for pharmacomechanical or mechanical clot dissolution. The AngioJet has a good track record with a 93% immediate improvement in treated arteries (PEARL Registry). Unique complications of the AngioJet include arrhythmias (when used near the heart), and hemolysis. Pharmacologic adjuncts are used in combination with these devices.
Open surgical embolectomy. In the setting of severe symptoms, open surgical techniques may be required. Treatments include balloon embolectomy with possible patch angioplasty or bypass grafting.
Additional Management Consideration
Systemic anticoagulation: If the embolus is noted incidentally, and is asymptomatic, determination of source is important in hopes of mitigating future adverse events. Systemic anticoagulation may be all that is necessary for long-term management.
Diagnosis
Embolus to the distal right common femoral artery.
Pearls
The medication tenecteplase has greater fibrin affinity. This lessens peripheral activation of circulating plasminogen, theoretically improving rates of distant bleeding complications.
After embolus removal, and establishment of flow, it is necessary to address the cause of the embolus. This may include systemic anticoagulation, antiarrhythmic drugs, or repair of aneurysms or ulcerative lesions.
Suggested Reading
Morrison HL. Catheter-directed thrombolysis for acute limb ischemia. Semin Intervent Radiol. 2006; 23(3):258–269Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree