Part 3 Aunt Minnies… because, sometimes, “it is what it is!”
Unlike previous parts, this part presents cases where the diagnosis is singular. In order to appease the “Top 3” gods, the author has presented each case with standard “Top 3 Discussion Points”: (1) What is it?, (2) How does it present?, and (3) What to do about it?; or, with procedures: (1) What it is?, (2) Why to do it?, and (3) How to do it?
Case 91
Clinical History
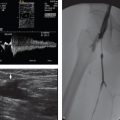
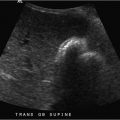
A 59-year-old man with abdominal pain and hypotension. Physical examination is remarkable for a pulsatile midline abdominal mass (Fig. 91‑1 ).

Key Finding
Rupture of an abdominal aortic aneurysm (AAA).
Top 3 Discussion Points
What is it? AAA rupture occurs when the bulging aorta is weakened to a point that it bursts, or leaks, resulting in internal bleeding, which is almost always life threatening.
How does it present? Frank rupture of an AAA is commonly a fatal event, with an overall mortality rate of about 90%. This is the classic surgical emergency! Patients may present with hypovolemic shock, back pain, and flank or periumbilical bruising (Grey Turner’s and Cullen’s signs, respectively). CT findings of periaortic stranding, retroperitoneal hematoma, and/or extravasation of contrast are solid signs of a ruptured AAA.
What to do about it? Immediate communication with the patient’s physician and surgical consultation are a must! Open control and repair of the aorta are typically required to save the patient; endovascular repair is sometimes adequate in select patients at experienced centers.
Additional Clinical Situations
Impending rupture: Of similar importance is identification of impending rupture of AAAs. Unlike patients with rupture, CT findings of impending rupture are more subtle. Signs include a rapid growth rate, luminal expansion with lysis of thrombus, high-attenuation crescent, discontinuity of intimal calcification, tangential calcium, the draped aorta, and penetrating ulcer.
High-attenuation crescent: The crescent represents acute blood within mural thrombus or aneurysm wall. Blood is usually asymmetrical and dense. There is often some adjacent retroperitoneal contained fluid. This sign has a specificity of 93%.
Changes in mural calcification: Atherosclerotic calcification is often seen within the wall of aneurysms. Focal discontinuity of otherwise circumferential calcified intimal plaque may indicate contained rupture. Two pieces of calcium with an altered angle from the expected wall perimeter (e.g., in the angle of a bulge), the so-called tangential calcium sign, can also indicate impending rupture.
The draped aorta: In this situation, the posterior wall of the aorta drapes along the contour of the adjacent vertebral body, often with indistinct fat planes. This may be due to weakening of the posterior wall versus a contained rupture.
Penetrating atherosclerotic ulcer: In this entity, wall disruption has extended through the intima and into the media. This can lead to intramural hematoma or saccular aneurysm formation.
Postrepair findings: Despite open or endovascular aortic repair (EVAR), rupture can still occur. Rupture is more likely in EVAR patients than in those with open repair. Imaging findings suggestive of post-EVAR instability include stent graft migration of greater than 5 mm, graft kinking or fracture, and persistent endoleak. Surgical grafts will normally have 20 to 30% fusiform dilation (polytetrafluoroethylene [PTFE] graft material). This should not be confused with graft failure. Anastomotic pseudoaneurysms occur at a rate of 0.2 to 15% and are important to report as they represent contained rupture requiring intervention.
Diagnosis
a) Ruptured abdominal aortic aneurysm.
b) Impending rupture of aneurysm.
Pearl
Published rates of post-EVAR rupture range from 0.4 to 1.2% (occurring 3 days to 85 months postsurgery). However, newer cohorts using more advanced stent grafts have shown even better rates (e.g., DREAM study) with no ruptures noted during 10-year follow-up.
Suggested Reading
Wadgaonkar AD, Black JH III, Weihe EK, Zimmerman SL, Fishman EK, Johnson PT. Abdominal aortic aneurysms revisited: MDCT with multiplanar reconstructions for identifying indicators of instability in the pre- and postoperative patient. Radiographics 2015;35(1):254–268Case 92
Clinical History
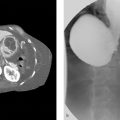
(a) A 79-year-old man in shock postprocedure day 1 from a left renal biopsy. (b) An 81-year-old man in shock with massive gastrointestinal (GI) bleeding (Fig. 92‑1).

Key Finding
CT and angiographic findings in hypotension.
Top 3 Discussion Points
What is it? On imaging, a specific constellation of findings has been dubbed the “CT hypotension complex.” The findings include “shock bowel,” flattening of the inferior vena cava (IVC), diminution of the aortic caliber, abnormal enhancement of the pancreas with associated peripancreatic fluid, and hypoperfusion of the liver and spleen. All of this is commonly seen in posttraumatic hypovolemia but can be seen in other causes of hypotension.
How does it present? Imaging of the hypotensive patient (of variable causes) leads to a number of signs. “Shock bowel” is most often seen on contrast-enhanced CT and is characterized by segments of small bowel thickening (> 3 mm wall) with extensive submucosal edema and concomitant hyperenhancement of the mucosa. CT will also sometimes demonstrate a slit-like vena cava (< 9 mm in anteroposterior [AP] diameter at level of renal veins), IVC “halo sign” (low-density fluid surrounding the IVC), small-caliber aorta, and variable enhancement of abdominal/retroperitoneal solid organs (e.g., “tigroid” spleen, variable pancreatic/hepatic enhancement, delayed nephrograms, and increased adrenal enhancement). On angiography, vessels are diffusely small in caliber with altered organ perfusion.
What to do about it? When visualized, the key to management is to understand the etiology and to ensure there is no confusion with alternative conditions with a somewhat similar appearance. For example, “shock bowel” needs to be differentiated from bowel injury and bowel infarction, the latter two of which require urgent surgical management instead of simple volume replacement. True posttraumatic “shock bowel” has been associated with an elevated trauma mortality rate, up to as high as 70%.
Diagnosis
Radiographic hypotension complex.
Pearls
Most commonly seen in posttraumatic hypovolemia, the “CT hypotension complex” is also associated with head or spine injury, cardiac arrest, septic shock, bacterial endocarditis, and diabetic ketoacidosis.
The collapsed IVC is more commonly seen in posttraumatic hypotension, when compared with the nontraumatic causes of the “CT hypotension complex” (where it is seen in < 40% of nontrauma cases).
Less commonly seen CT signs of hypovolemia include “shock thyroid” (with fluid around the thyroid), and the “black kidney sign” (absent renal enhancement in posttraumic pediatric patients).
Differentiating bowel injury from “shock bowel” is aided by the focality of bowel injury (“shock bowel” is more diffuse), and the presence of high-density blood in the wall (“shock bowel” has low-density edema).
Both bowel injury and bowel ischemia do not have mucosal hyperenhancement, unlike “shock bowel.”
Suggested Reading
Ames JT, Federle MP. CT hypotension complex (shock bowel) is not always due to traumatic hypovolemic shock. AJR Am J Roentgenol 2009;192(5):W230–W235Case 93
Clinical History
A 38-year-old woman with a painful and swollen left leg (Fig. 93‑1).

Key Finding
Extraluminal compression of left common iliac vein by the right common iliac artery.
Top 3 Discussion Points
What is it? May–Thurner syndrome is the collection of left leg symptoms (typically swelling with or without thrombosis) associated with the anatomic finding of extrinsic compression of the left common iliac vein by the crossing of the right common iliac artery.
How does it present? The classic patient is a woman in the second to fourth decade with otherwise unexplained left leg swelling. Other left leg symptoms may be varicose veins, deep vein thrombosis, chronic venous stasis changes (e.g., skin thickening, scaling, ulcers), or even phlegmasia cerulea dolens (impaired blood flow in the leg due to extensive venous thrombosis, leading to limb threat). Rarely, deep vein thrombosis associated with May–Thurner may present as pulmonary embolus. Imaging with CT or MR venography or conventional venography is best, as Doppler ultrasound will often be unable to visualize the common iliac vessels. On conventional venography, the presence of an extrinsic compression at the classic level, with pelvic collateral veins, and a pressure gradient help diagnose May–Thurner and direct management.
What to do about it? Venous compression syndromes are difficult clinical situations. Excluding a pelvic or retroperitoneal mass is wise. Treatment is offered only when individuals are symptomatic. In May–Thurner, a number of surgical approaches have been developed, but lack general acceptance. Endovascular techniques have a role, especially thrombolysis of associated deep vein thrombosis in young/able patients. After thrombolysis, both angioplasty and stent placement have been used with variable success. The goal is to palliate symptomatic individuals via durable effacement of the stenosis, directing flow away from collaterals, and reducing any supine venous pressure gradient to less than 1 to 2 mm Hg.
In acute May–Thurner affecting young and functional patients, or those with phlegmasia, with clot less than 14 days old, pharmacologic or pharmacomechanical thrombolysis is most commonly used. As the thrombus organizes and ages, lytic therapies are less efficacious (e.g., chronic clot is defined as greater than 4 weeks old). After thrombolysis, or in the setting of May–Thurner without clot, balloon venoplasty followed by stent placement is the generally accepted treatment of the day. Most use larger stents (typically 12–16 mm in diameter). Patency rates of 80 to 100% over 1 to 2 years, even up to 6-year follow-up, have been reported (slightly less patency in those presenting with thrombus-associated May–Thurner). Bleeding complications during thrombolysis and stent placement occur in 5 to 15% of patients, with most bleeding occurring at the venous access site or within the abdomen. Intracranial bleeding is rare, occurring about 0.2% of the time. The rate of pulmonary embolus during thrombolysis is about 1%. Because of this risk, some operators prefer to place an IVC filter during fibrinolytic procedures.
After opening the vessel, systemic anticoagulation is typically necessary (of variable duration depending on history). Additionally, stent patients should be placed on dual antiplatelet therapy, like aspirin and clopidogrel, for 3 to 6 weeks to prevent early stent occlusion.
Diagnosis
May-Thurner syndrome.
Pearls
May–Thurner is also known as iliac vein compression syndrome, Cockett’s syndrome, and iliocaval compression syndrome.
May–Thurner is diagnosed in 2 to 5% of people being evaluated for chronic venous disorders of the left leg.
Suggested Reading
Cil BE, Akpinar E, Karcaaltincaba M, Akinci D. Case 76: May–Thurner syndrome. Radiology 2004;233(2):361–365Case 94
Clinical History
A 38-year-old man with chronic unilateral hand pain and intermittent pallor (Fig. 94‑1).

Key Finding
Hypothenar hammer syndrome.
Top 3 Discussion Points
What is it? Hypothenar hammer syndrome is due to repetitive trauma at the distal ulnar artery, near the hook of the hamate, resulting in dissection, stenosis/occlusion, or pseudoaneurysm formation. Vessel injury can lead to distal thromboembolic disease, affecting the digital arteries along the ulnar side.
How does it present? Patients present, often to primary care providers, with hand and finger pain. Many are initially misdiagnosed with overuse syndromes. Most patients are men, mean age of 40 years, with an occupational or recreational history congruent with repetitive trauma of the hypothenar portion of the hand. The repetitive blunt trauma leads to intimal injury, vasospasm, and promotes platelet aggregation. The injury may continue through other layers of the arterial wall, leading to aneurysm formation. With clot formation, emboli are often delivered to the digital arteries, resulting in ischemia. Because of the variability of the palmar circulation, the degree of ischemia is unpredictable from patient to patient and may be subtle to severe. Digits most commonly affected are from the index to little finger (ulnar aspect of the hand). Patients often have pain, but may have paresthesias, cold sensitivity, or cyclical discoloration of the fingertips (Raynaud’s like, minus the erythematous phase). On physical examination, callous is often noted involving the hypothenar soft tissues, typically of the dominant hand. Rarely, there is a pulsatile mass in the setting of aneurysm formation. In its most severe form, patients may suffer from digital tissue loss. Although Doppler ultrasound is being used to suggest the diagnosis, angiography is the best examination. Findings on angiography include ulnar tortuosity (typically at the site of injury), aneurysm, ulnar artery occlusion, digital artery occlusion, and presence of thrombi/emboli.
What to do about it? Treatment is offered based on the degree of symptoms and vessel changes. Conservative management involves smoking cessation, removing the repetitive traumatic practice, protective/padded hand wear, and avoiding provocative situations (e.g., cold). Pharmacologic measures include a calcium channel blocker, antiplatelet agents, systemic anticoagulation, and pentoxifylline (reducing blood viscosity). With acute, severe ischemia, surgery may be needed. Options include ulnar artery ligation (must have intact radial palmar arch), resection of the diseased segment with end-to-end anastomosis, or resection with vascular graft reconstruction. Superiority of surgery over conservative measures has not been determined given variable vessel injury and presentation, along with small cohorts.
Diagnosis
Hypothenar hammer syndrome.
Pearls
Hand symptoms caused by hypothenar hammer syndrome may be confused with other diseases—Raynaud’s disease, Raynaud’s phenomenon associated with connective tissue disease, trauma (including heat and cold injuries), vasculitis, throbmoangiitis obliterans, or emboli from other arterial sources.
Although rare in the general population, hypothenar hammer
was noted clinically, and by Doppler, in 14% of auto mechanics who shared a history of habitual hammering.
Suggested Reading
Ablett CT, Hackett LA. Hypothenar hammer syndrome: case reports and brief review. Clin Med Res 2008;6(1):3–8Case 95
Clinical History
A 41-year-old man with right hand pain and a blue thumb (Fig. 95‑1).

Key Finding
Thoracic outlet syndrome.
Top 3 Discussion Points
What is it? Thoracic outlet syndrome is a constellation of clinical and anatomic/radiographic findings related to compression of the brachial plexus, subclavian artery, and the subclavian vein as they exit the “thoracic outlet.” The outlet is defined as the space cephalad to the first rib, and posterior to the head of the clavicle, encompassing the interscalene triangle, the costoclavicular space, and retropectoralis minor space. Compression is most likely to affect the nerves (95% of cases), followed by the vein (4%) and the artery (1%). The compression may be due to anatomic structures (e.g., cervical ribs, elongated transverse process of the C7 vertebrae, aberrant scalene musculature) or changes post-trauma (e.g., excessive callus after first rib or clavicular fractures).
How does it present? The typical patient is a young woman, between the age of 20 and 40 years old. Symptoms are most often manifest during sustained elevation and abduction of the arm (e.g., painting overhead). Most symptoms are related to brachial plexus compression, affecting the C8 and T1 nerve roots, leading to pain and paresthesia in the ulnar distribution. Alternatively, C5–C7 may be compressed, leading to similar symptoms, but involving the radial distribution (or the neck, ear, upper chest, and back). Neurologic compression is difficult to diagnose and treat. Compression of the vein may lead to thrombosis presenting as arm swelling, cyanosis, and pain. Arterial compression may cause thrombosis, stenosis, or aneurysm formation, leading to distal ischemia or emboli, presenting as pain, coldness, pallor, paresthesia, and rarely tissue loss. Imaging with Doppler ultrasound, MR, CT, and conventional angiography are complementary, and are often used in conjunction with one another to fully understand all causal factors.
What to do about it? Management is dependent on cause and severity of symptoms. Neurologic compression is most often treated conservatively: physical therapy, anti-inflammatory medications, and muscle relaxants. Venous thrombosis usually requires catheter-directed thrombolysis and anticoagulation. Surgical thoracic outlet decompression may be necessary. Arterial compression may be severe and present as acute thrombosis. Urgent restoration of blood flow may require catheter-directed or open interventions, followed by systemic anticoagulation. After restoration of flow, or in the setting of nonthrombotic disease, thoracic outlet decompression surgery may be required (e.g., cervical rib or first rib resection, removal of anterior scalene) with or without resection and repair of any concomitant aneurysm. Primary stent placement of an arterial stenosis is not optimal, but is reserved for recurrent stenosis after open decompression surgeries.
Diagnosis
Arterial thoracic outlet syndrome.
Pearls
Narrowing at the thoracic outlet may occur at rest or in the neutral arm position or be exacerbated by physical maneuvers such as abduction and external rotation of the arm.
Vascular compression is most likely to be due to narrowing at the costoclavicular space, and less likely in the interscalene triangle. Neurologic compression occurs equally in these two spaces. The retropectoralis minor space is rarely the site of compression.
Estimates of 0.3 to 8% of the population may suffer the effects of thoracic outlet syndrome.
Both limbs should be interrogated during work-up as up to 15% of patients will have bilateral findings.
Suggested Reading
Eliahou R, Sosna J, Bloom AI. Between a rock and a hard place: clinical and imaging features of vascular compression syndromes. Radiographics 2012;32(1):E33-E49Case 96
Clinical History
A 22-year-old woman with left arm swelling after traction injury while walking the dog (Fig. 96‑1).

Key Finding
Paget–Schroetter syndrome.
Top 3 Discussion Points
What is it? Paget–Schroetter is a subtype of venous thoracic outlet syndrome, caused by vigorous overhead arm activity. The commonality to the inciting movement includes extremity retroversion, extension, and hyperabduction. These motions pull on the subclavian vein leading to microtrauma to the endothelial lining, and initiating coagulation. The presence of an anatomic abnormality to the thoracic outlet increases the likelihood of thrombosis, due to stasis of blood, and in acting as a tether during traumatic motions. A repetitive cycle of trauma, and thrombosis, leads to intimal hyperplasia, inflammation, and fibrotic stenosis or occlusion.
How does it present? Most patients are young, athletic males. They most often present with acute or subacute arm pain and swelling (most often the dominant arm), usually after sporting activities such as gymnastics, swimming, and wrestling. Less common symptoms include arm heaviness, redness, and cyanosis. There is a risk of pulmonary embolus. On physical examination, visible, dilated collateral veins may be seen along the upper arm and chest (Urschel’s sign). Imaging typically begins with Doppler ultrasound, with conventional, CT, or MR venography used for more complete evaluation after initial screening. Advanced axial imaging can be extremely helpful in planning thoracic decompression.
What to do about it? Although there is no consensus for management, due to the rarity of the disease, most advocate for thrombolysis and surgical decompression in symptomatic patients. Simple conservative measures, using anticoagulation and limb elevation, have a high degree of residual symptoms and disability (perhaps because it affects athletes who are sensitive to more minute changes in ability). Thrombolysis is likely to require a catheter-directed infusion over 24 to 48 hours. Thoracic outlet decompression usually follows. Postdecompression residual disease is most often addressed via endovascular techniques, including venoplasty and/or stent placement. The need for systemic anticoagulation is debatable, typically dependent on diagnosis of a hypercoagulable state or patients with recurrent thrombosis.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree