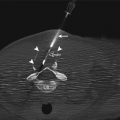
Fig. 8.1
Direct smear prepared from FNAB sample of a lung mass and stained using the Papanicolaou method. Note the presence of many tumor cells with prominent nucleoli. The presence of melanin pigment in one tumor cell confirms the diagnosis of metastatic melanoma
The needle used for aspiration can be rinsed in solutions such as RPMI medium 1640 (Gibco, Invitrogen) or CytoLyt solution (Hologic Corp.) immediately after the aspirate has been added to glass slides; this approach is useful for preparing tissue blocks, also referred to as cell blocks. Cell blocks are useful for collecting any remaining cells and tissue fragments in the needle and syringe that can be used for additional cytopathologic evaluation. The RPMI or CytoLyt solution is then centrifuged to obtain a sediment containing the cellular material. A small amount of sediment is used to prepare one or two cytospin smears, which can be stained similarly to a direct smear. Cell blocks can be prepared if sufficient sediment is available. Different methods are used to prepare cell blocks [2–5]. One of the most common methods includes wrapping the sediment in Shark Skin Filter Paper (Whatman); this is then placed in a plastic cassette, fixed in formalin, and subjected to routine processing. After processing, the cell blocks are embedded in paraffin wax to create tissue blocks, which are cut at 5-μm intervals and stained using the H&E method for pathologic interpretation. These cell blocks are a valuable resource for generating additional unstained sections for ancillary studies using methods such as immunocytochemical analysis, in situ hybridization, or polymerase chain reaction (PCR) or next generation sequencing.
Core Needle Biopsy (CNB)
CNB is performed using a 14–21-gauge, coaxial, automated cutting needle biopsy system (Cook). Usually, 2–4 cores of tissue are obtained from the lesion. For conventional pathological examination, the samples are fixed in formalin, routinely processed, and embedded in paraffin. The paraffin blocks are then cut at 5 μm and stained with H&E for final histopathological interpretation. The advantage of CNBs over FNAB is the availability of tissue architecture; this facilitates the rendering of a specific diagnosis on the basis of histopathologic examination, alone or in conjunction with ancillary testing. The entire process, from fixing the sample in formalin to the availability of tissue sections for pathologic interpretation, can take a mean of 6–24 h. Therefore, CNBs are not available for immediate assessment, unlike FNAB, in which direct smears are available within a few minutes of the procedure to determine specimen adequacy, localization accuracy, and a preliminary diagnosis. However, in centers in which immediate evaluation by cytopathologists or cytotechnologists is available, touch imprints of CNBs can be prepared by gently touching the cores on glass slides. These touch imprints can be fixed in alcohol for Papanicolaou or H&E staining and air-dried for Diff-Quik staining, similarly to direct FNAB smears, for immediate assessment of specimen adequacy and to determine whether the CNB represents the target lesion [6, 7].
FNAB and CNB are complimentary techniques for evaluating the lesion of interest; both have distinct advantages and limitations. In centers in which immediate assessment of the specimen is possible, direct FNAB smears or touch imprints can contribute to the overall success of obtaining diagnostic material from the target lesion. Direct smears may be more useful than touch imprints for rendering a preliminary diagnosis within a few minutes of the biopsy. FNAB is the preferred method in technically challenging sites such as those close to major blood vessels or neurovascular bundles. In addition, FNAB is the preferred method for obtaining material for microbiologic culture studies from lesions that are thought to be infectious on the basis of clinical history, radiologic images, the results of direct FNAB smears, or CNB touch imprints. In cases of suspected hematopoietic neoplasms, FNAB is better than CNB for obtaining material for immunophenotyping by flow cytometry for establishing the clonality of the lymphoid cells [8]. The cytomorphological features alone are sufficient to render a specific diagnosis in many cases. The availability of tissue architecture in FNAB cell blocks aids in generating a specific diagnosis. However, tissue architecture in larger CNB fragments eases interpretation and the rendering of a definite and specific diagnosis. CNB samples invariably contain more tissue than do FNAB samples, making ancillary studies possible. In selected cases, either technique alone can be used, but both are preferable because both have distinct diagnostic advantages. Figure 8.2 shows a case of CT-guided biopsy of a 2.5 cm lung mass; diagnostic material was found on an FNAB direct smear and cell block and on CNB. Figure 8.3 illustrates a case of sonography-guided FNAB and CNB of an enlarged neck lymph node for which CNB, but not FNAB, provided diagnostic material.
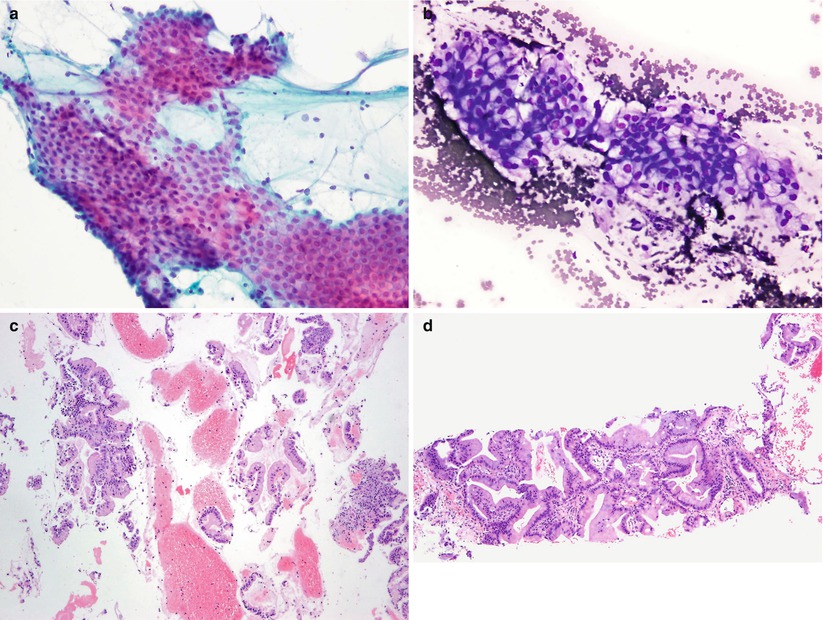
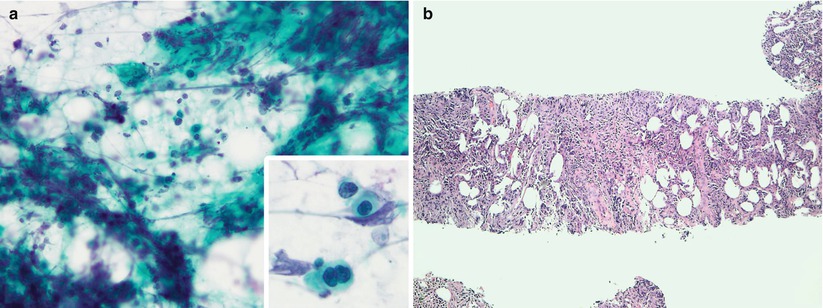

Fig. 8.2
Direct smears prepared from FNAB of a lung mass, stained using the Papanicolaou (a) and Diff-Quik (b) methods, reveal mucin-containing tumor cell fragments in the cytoplasm. The corresponding cell block section (c) reveals several fragments of tumor cells that are consistent with mucinous bronchioloalveolar carcinoma. The core needle biopsy of the lung mass is shown in (d). Note that the concurrently obtained CNB sample demonstrates the same tumor in a larger continuous tissue fragment

Fig. 8.3
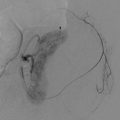
Direct smears prepared from FNAB of an enlarged neck lymph node stained using the Papanicolaou method (a) reveal a prominent crush artifact, with only a few scattered atypical cells. The corresponding CNB sample (b) reveals adequate diagnostic material that permits accurate pathologic evaluation of the tissue
Several investigators have compared the performance of FNAB and concurrent CNB for evaluating target lesions in selected organs [9–18]. Most of these studies concluded that both these techniques are generally complementary. The diagnostic yield may be similar for both techniques, but in a small number of patients, diagnostic material can only be obtained using one or the other method. Therefore, overall diagnostic accuracy can be increased using both techniques, if possible, in any given patient. In most cases, the combination of FNAB and CNB can obviate the need for incisional biopsy or other invasive procedures, such as mediastinoscopy, thoracoscopy, or laparoscopy; all of these procedures are performed under general anesthesia and are generally associated with higher morbidity and cost than are preoperative biopsies performed in radiology or pathology suites.
Ancillary Testing
Ancillary tests, when used in conjunction with conventional cytopathological or histopathological examination, increase the specificity of the pathologic diagnosis. The different ancillary tests that can be used in anatomic pathology are described in the sections below.
Immunohistochemical Analysis
Immunohistochemical analysis is the most commonly used ancillary test in the work-up of specimens; it plays a valuable role in rendering a specific pathologic diagnosis. The technique entails the use of antibodies specific to pertinent epitopes in cells. The antibody binds to the epitope, which, if present in the tissue, can be visualized using different types of chromogens [9]. Immunostaining can be performed using tissue obtained by CNB or FNAB, provided the tissue contains adequate material. Because more representative tissue is available on CNB than on FNAB, CNB is preferred for immunostaining. However, with good-quality cell blocks that contain adequate representative tissue, the results of immunostaining with FNAB cell block sections can be equivalent to those of CNB sections. Cell blocks are the optimal FNAB preparation for immunostaining, but alcohol-fixed or air-dried smears and formalin-fixed direct smears can be used when cell blocks, or CNB samples are not available. Because the positive control used for immunostaining of smears is usually formalin-fixed tissue and not a similarly prepared direct smear, careful interpretation of the results is generally recommended. Similarly fixed direct smears should be used as positive controls to validate antibodies’ performance in direct smears compared with formalin-fixed, paraffin-embedded tissue; this should be established before the direct smears are used for immunostaining.
Common applications of immunohistochemical analysis include detecting infectious agents; categorizing and typing tumors; determining the site of origin of metastatic tumors, including those with multiple or unknown primaries; and evaluating prognostic and predictive tumor markers for targeted therapy. Antibodies against the intermediate filaments of cells, namely, cytokeratins in epithelial cells and vimentin in mesenchymal cells, facilitate the categorization of poorly differentiated tumors, as carcinoma when positive for the former marker and as sarcoma when positive for the latter. The expression patterns of different types of low- and high-molecular weight cytokeratins are specific and consistent with respect to organs. Primary antibodies developed against specific cytokeratins are valuable for phenotyping benign and malignant tissues to determine the organ of origin. The use of a panel of two cytokeratins, CK7 and CK20, can help in determining the site of origin of many epithelial tumors [19]. The organs and their corresponding tumors that are positive for CK7 but negative for CK20 include the lungs, breasts, and ovaries; those that are positive for CK20 but negative for CK7 include the colorectum and urinary bladder; those positive for both markers include selected instances of tissues from the stomach and pancreaticobiliary tract; and those negative for both include the kidneys, adrenal gland, and liver. In addition, several specific antibodies are available to help further characterize tumors.
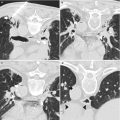
Tissue specific markers include thyroid transcription factor 1, surfactant apoprotein A, and Napsin A for lung adenocarcinoma; mammaglobin, gross cystic disease fluid protein 15 (GCDFP15), and estrogen and progesterone receptor expression for mammary tumors; HePar1 and glypican 3 for hepatocellular carcinoma; prostate-specific antigen, prostate-specific acid phosphatase, and prostein for prostate disease; parathyroid hormone for the parathyroid gland; thyroglobulin and thyroid transcription factor 1 for the thyroid gland; renal cell carcinoma marker and CD10 for renal cell carcinoma; and calretinin, D2-40, and mesothelin for mesothelial cells [20–22]. Markers for confirming neuroendocrine differentiation include chromogranins, synaptophysin, and CD56 [23]. Markers for diagnosing melanoma include S-100 protein, MART1, Melan A, HMB45, and tyrosinase [24]. Prognostic and predictive markers that can be evaluated by immunohistochemical analysis include estrogen and progesterone receptors and human epidermal receptor 2 (HER2)/neu in breast carcinomas and c-kit in gastrointestinal stromal tumors [25–27]. Figure 8.4 shows a poorly differentiated carcinoma on a sonography-guided CNB of a 2.5-cm mass in the liver that is positive for estrogen receptor and demonstrates HER2/neu protein overexpression on immunohistochemical analysis.
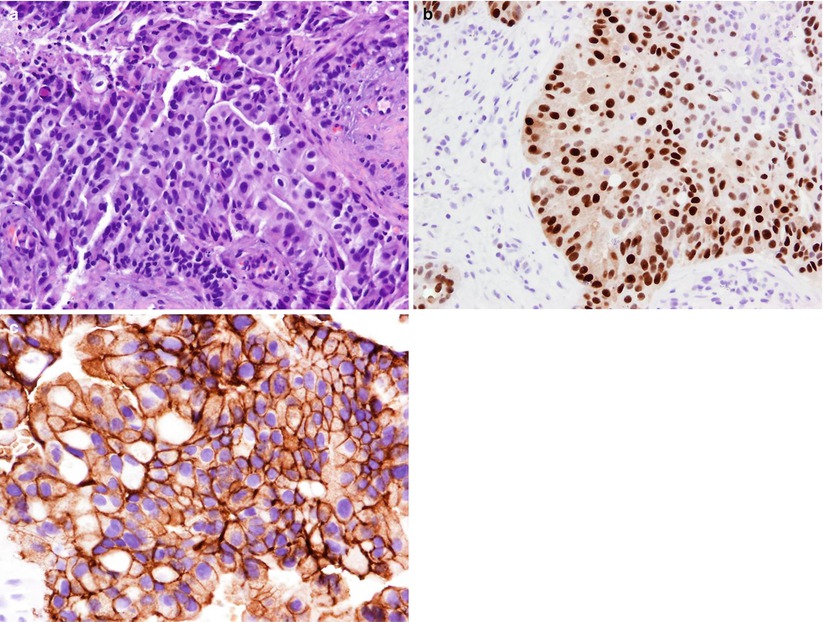

Fig. 8.4
Tissue section prepared from CNB of a liver mass stained using the H&E method (a) demonstrate features of a poorly differentiated adenocarcinoma. The tumor cells are positive for estrogen receptor (b) and HER2/neu on immunohistochemical staining (c). The overall findings support the diagnosis of metastatic breast carcinoma
Leukocyte common antigen is an immunomarker for hematopoietic cells [28, 29]. CNB is optimal for immunostaining in hematopoietic lesions; however, selected immunostains can be performed on cytospin FNAB smears to characterize lymphoid cells. The most common B cell markers are CD19, CD20, CD79, PAX5, and kappa and lambda light chains; T-cell markers include CD2, CD3, CD5, CD7, CD4, and CD8. For typing lymphomas, useful markers are bcl2 for follicular lymphoma, cyclin D1 for mantle cell lymphoma, ALK1 for anaplastic lymphoma, and CD138 for plasma cell tumors [28, 29]. CNB is preferred for diagnosing non-Hodgkin’s lymphoma; FNAB can be equivalent using a multiparametric approach, including cytomorphological evaluation and ancillary immunophenotyping by flow cytometry [30]. Figure 8.5 shows the results of ancillary immunostains performed on unstained CNB sections of the case in Fig. 8.3. The tumor cells are positive for leukocyte common antigen, CD20, and lambda light chain. The overall cytomorphological features, in conjunction with ancillary immunostains, are consistent with high-grade malignant B cell lymphoma. It is to be noted that in patients with Hodgkin disease, CNB is optimal because fibrosis makes it difficult to obtain cells and because a panel of immunostains is needed to establish the diagnosis.
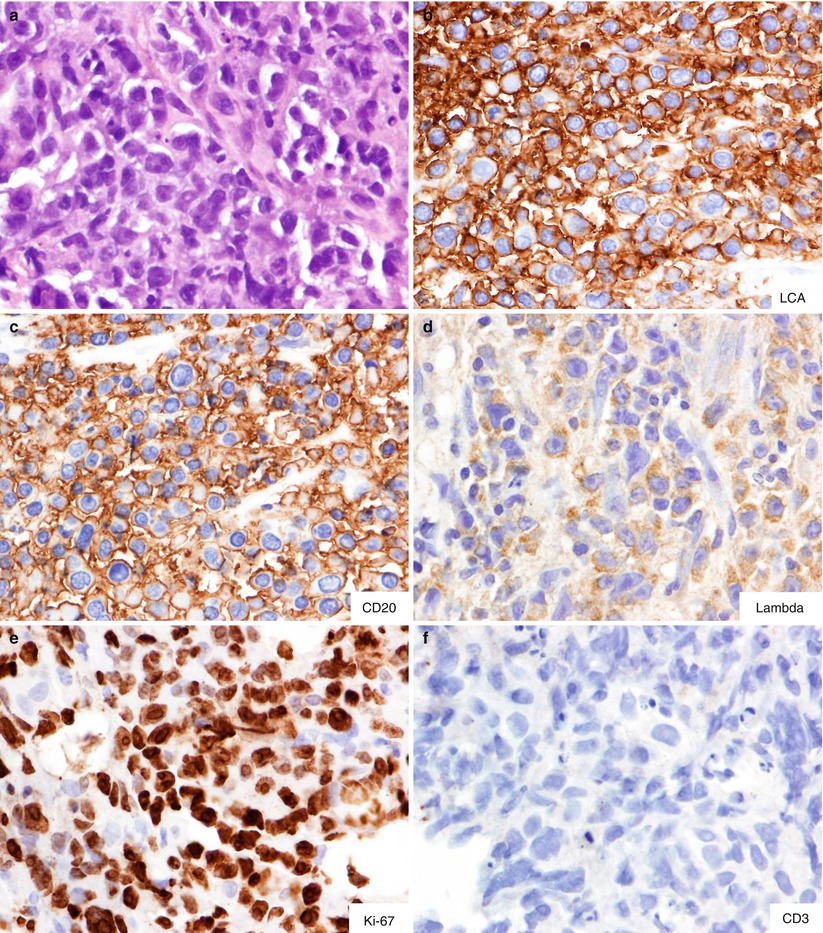

Fig. 8.5
Ancillary immunostains performed on the CNB of the case in Fig. 8.3. H&E stain shows large cell lymphoma involving the lymph node (a). The tumor cells are positive for leukocyte common antigen (b), CD20 (c), and lambda light chain (d) and negative for CD3 (f). Note the high proliferative index, as demonstrated by Ki67 labeling, in almost all tumor cells (e). The overall findings are consistent with high-grade malignant B cell lymphoma
Flow Cytometry Analysis
Immunophenotyping by flow cytometry analysis is routinely performed on all suspected hematopoietic lesions to characterize the cells comprising the lesion and to establish clonality. While both CNB and FNAB samples can be used, but the latter is preferred because the cells are already in suspension [8]. However, with FNAB, it is important to ensure that the aspirate contains an adequate number of cells. Immediate assessment of direct smears for the presence of the cells of interest and the extent of blood contamination can be useful for determining the quality of the aspirate for successful immunophenotyping. However, counting the lymphoid cells in an RPMI suspension using a cell counter can provide more definite proof. Approximately 5–10 million cells are generally adequate for flow cytometry analysis of a variety of T and B lymphocytic or myeloid cell panels that can be tested on the basis of the preliminary diagnosis of direct smears or CNB touch preparations.
Molecular Testing
Several molecular tests are performed as the standard of care to arrive at a definite diagnosis or to select patients for or predict response to targeted therapy. The list of molecular markers that can be tested is rapidly evolving. CNB specimens are preferred for molecular tests such as in situ hybridization, PCR, sequencing, and transcriptional profiling on preoperative tissue obtained under imaging guidance. However, FNAB samples, including alcohol-fixed, air-dried direct smears; aspirate in any transport media; and unstained sections of formalin- or alcohol-fixed, paraffin-embedded cell blocks can also be used provided they demonstrate adequate cellularity. The details of the molecular tests and the optimal specimen needed for each test are summarized in the sections below.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree