Etiology
Mayo Clinic
(1965)a (%)
University of Minnesota
(1979–1983)b (%)
London
(1976–1979)c (%)
Mayo
(1990)d (%)
AFIP
(1990–1997) (%)
Toronto
(2008)e (%)
Bicuspid
49
49
56
36
30
32
Post-rheumatic
33
23
24
9
13
11
Tricuspid degenerative
0
28
12
51
49
64
Unicuspid
10
1
0
0
6
3
Other
7
0
8
2
2
1
In general, the age at presentation is quite predictive of the underlying pathologic lesion [3]. Unicuspid aortic valve occurs in 0.02 % of the population and presents in childhood, adolescence, or early adulthood and overall accounts for only 4–6 % of CAS. In contrast, bicuspid aortic valve has a prevalence of 1–2 % in the general population, a male preponderance ratio of 2:1, and is responsible for 30–50 % of surgically removed calcified aortic valves. Patients with bicuspid CAS present at an earlier age (45–55 years of age) than patients with tricuspid CAS (65–75 years) [4]. Tricuspid CAS is present in approximately 1–2 % of patients older than 65 years and 4 % of patients >85 years with men affected more commonly than women (male to female ratio = 1.6:1). With the aging of the general population in the USA, tricuspid CAS is responsible for nearly 60 % of surgical valve replacements with most patients being close to 70 years of age [2, 5].
Aortic sclerosis and stenosis are considered to represent a spectrum of the same disease process with calcification as the final common pathway leading to stenosis. While the rate of progression from mild to severe stenosis tends to be highly variable among individual patients [6], a 7-year echocardiography study observed a rate of progression to AS of any degree of approximately 16 % in elderly patients (age 70.8 ± 7.8 years) with aortic sclerosis [7]. Early sclerotic changes are characterized by fibrotic thickening and early calcification of the valve cusps without left ventricular outflow obstruction. Such changes are common in elderly patients (20–30 % in those >65 years and 48 % >85 years) and are not usually associated with a significant gradient across the valve. Aortic sclerosis however has important clinical implications beyond its identification as a precursor lesion to CAS; namely, it is associated with a 50 % increased risk of death from cardiovascular disease and myocardial infarction even in the absence of hemodynamically significant aortic valve obstruction. Whether aortic sclerosis is a marker for increased risk of cardiovascular events or has a direct effect on clinical outcomes is not known. Interestingly, in patients with mitral annular calcification, aortic valve sclerosis is observed in nearly 50 % of cases, however, CAS is only observed in 17 % [8].
A recent echocardiographic study has found that hemodynamic progression of aortic stenosis in elderly patients is dependent on underlying baseline AS severity [9]. Mean aortic valve gradients increased an average of 4, 6, and 10 mmHg/year in elderly patients with mild, moderate, and severe AS, respectively. Overall, 74 % of patients with moderate AS and 35 % of patients with mild AS progressed to severe AS. Other predictors of rapid hemodynamic progression included advanced aortic valve calcification, severe chronic renal failure, and anemia.
Severe aortic stenosis results in a pressure overloaded left ventricle with concentric left ventricular hypertrophy and normal left ventricular chamber size. Once symptomatic, aortic valve replacement with either a mechanical or bioprosthetic valve is the only definitive treatment and is usually curative. The average survival without treatment is only 2–3 years with an increased risk of sudden death.
Pathology of Calcific Aortic Stenosis
Calcific Aortic Stenosis (Tricuspid)
The normal aortic valve is composed of three semicircular cusps (left, right, and posterior (noncoronary)) attached to the aorta at three commissures. The cusps are thin and translucent with a thickness of less than 1 mm and two surfaces lined by endothelial cells including the ventricular surface facing the left ventricle (inflow surface) and the aortic surface (outflow surface). The aortic valve cusp has three distinct histologic layers each of which is adapted to a specific function. These layers are defined as (1) the ventricularis, a loose connective tissue layer rich in elastic fibers on the ventricular side of the cusp; (2) the spongiosa, in the middle of the cusp, composed predominantly of proteoglycans and glycosaminoglycans; (3) the fibrosa, a dense collagen layer that extends on the aortic surface. Finally, valvular interstitial cells (VICs) constitute the majority of the cells in all layers of the valve and maintain the matrix of the normal valve tissue.
We and others have described the gross and histologic features of aortic valves at autopsy as well as valve cusps removed surgically. Grossly, tricuspid CAS demonstrates calcified masses and nodules involving the aortic surface of the valve cusps in an aortic valve that was previously anatomically normal. The earliest calcification occurs at cusp attachment sites (base of cusps) and extends along the line of closure or coaptation zone. Pathologic changes may range from mild cuspal thickening in early disease (aortic sclerosis) to end-stage stenotic lesions with calcific nodules filling the involved sinuses of Valsalva (Fig. 11.1). It has been shown that the distribution of calcium in stenotic cusps is not uniform and may differ considerably between individual cusps. In one study, surgically excised cusps showed differences in weights with one cusp differing from the other two (by >0.1 g) in 61 % of valves, whereas 26 % of valves demonstrated weight differences between all three cusps. Weight differences appeared to be caused by differing quantities of calcium [10]. Interestingly, calcification is typically more pronounced in the noncoronary cusp compared with the coronary cusps. One explanation for this finding may relate to the relatively reduced diastolic pressure loads around the coronary cusps due to the presence of the coronary ostia.
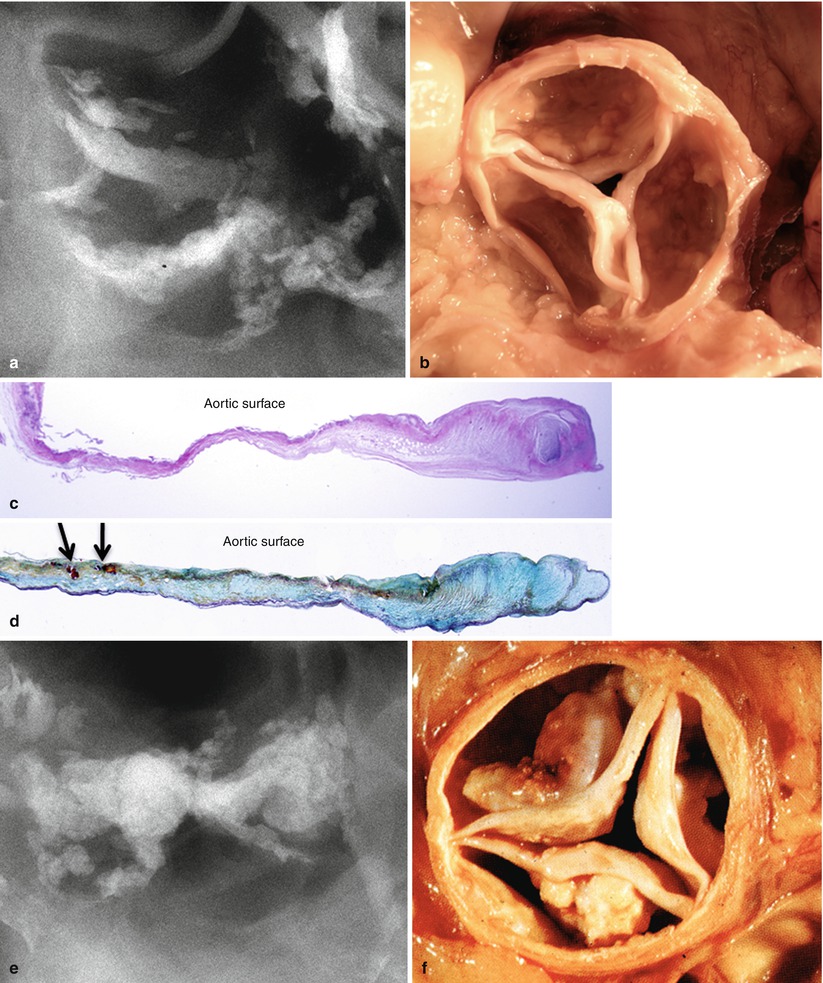
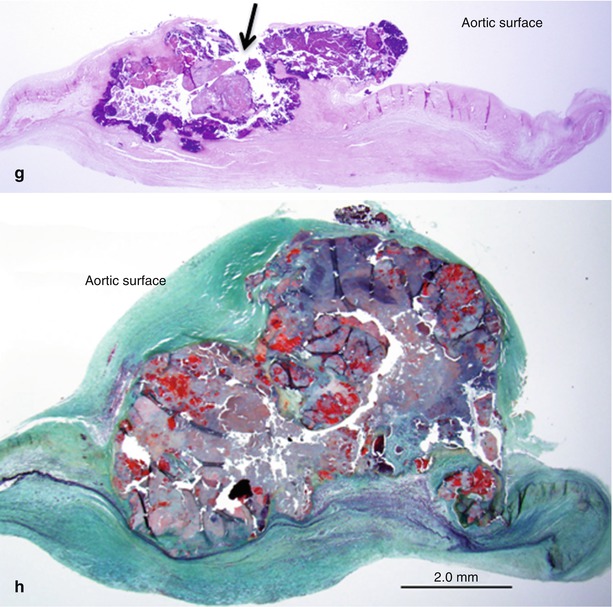


Fig. 11.1
Two aortic valves seen at autopsy showing progression of calcific aortic stenosis. (a, b) Radiograph and gross image of aortic valve from an 83-year-old female. This valve shows mild calcification with small calcific deposits on aortic surface of cusps and sparing of the free edge. (c, d) Histologic sections of cusps showing fibrotic thickening and microscopic calcific deposits in the zona fibrosa (arrows). (e, f). Radiograph and gross image of stenotic aortic valve from an 82-year-old female. There is marked nodular calcification of the cusps with bulky deposits on the aortic surface. (g, h) Histologic sections of the cusps show calcific nodules superimposed on a fibrotic cusp with ulceration of the aortic surface (arrow). Note fibrotic thickening of the ventricular surface
Histologically, early calcification—highlighted by von Kossa staining—appears as fine stippled calcifications or small nodular concretions typically located in the fibrosa layer on the aortic aspect of the valve cusp, especially in areas of early atherosclerotic change. The ventricular surface of the valve is mostly smooth exhibiting varying degrees of fibrosis. The “hinge” area of the aortic cusps near the attachment to the aortic root at an area just below the line of valve closure are regions where early calcific deposits are found and may be related to the higher mechanical forces in these regions (Fig. 11.2) [11]. Such site-specific vulnerability to calcification has also been partially attributed to differential endothelial gene expression on the aortic versus ventricular side of aortic valve cusps. It has been shown that the fibrosa on the aortic side of the cusp displays a “calcification permissive” transcriptional profile characterized by overexpression of genes related to skeletal development, vascular calcification, and bone formation such as bone morphogenetic protein 4 (BMP4) along with decreased expression of multiple inhibitors of cardiovascular calcification [12].
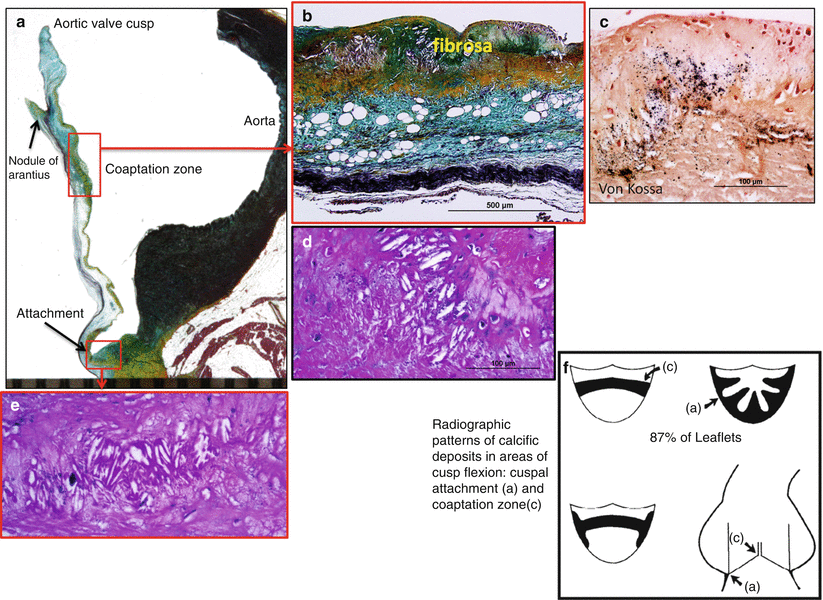

Fig. 11.2
Pattern of calcification in aortic valve cusps. (a) Longitudinal section of intact aortic valve cusp and aorta. The red boxes highlight areas of early atheromatous change with microscopic calcification in two regions: (1) the upper box shows an early lesion on the aortic surface at the coaptation zone and (2) an early lesion at the cuspal attachment site (lower box). Higher magnifications show fibrosa layer of cusp with atheromatous changes characterized by cholesterol clefts, lipid pools, and rare chronic inflammation (b, d, e). (c) Von Kossa staining highlights microscopic calcifications (calcium stains black). The diagram in (f) highlights radiographic patterns of calcific deposits in areas of cusp flexion. (c) Area of coaptation and (a) cusp attachment site. These radiographic areas mirror histologic regions of calcification (The diagram F was reproduced with permission from Thubrikar et al.[11])
As calcification progresses, there is coalescence of microcalcifications into larger complex plates and nodules, sometimes associated with surface ulceration. In a pathologic study of 247 surgically excised aortic valves (age range 17–86 years, mean 64.1 ± 13.4 years), morphologic features of aortic stenosis were present in almost 70 % of valves. Ulceration of calcific nodules was present in approximately 40 % of valves showing severe calcification [13]. In later stages, the calcification is often superimposed on a thickened and fibrotic cusp and there may be histologic evidence of bone matrix formation, osteocytes, and marrow elements.
Congenital Bicuspid and Unicuspid Valve
A congenital bicuspid aortic valve is a common congenital cardiac anomaly present in 1–2 % of the general population. Valvular stenosis is the most frequent physiologic complication affecting approximately 80 % of such valves studied at autopsy or post-valve replacement. Fifteen percent of congenital bicuspid aortic valves are surgically excised for insufficiency and only 5 % for combined aortic stenosis and aortic insufficiency [2].
Recently, macroscopic and microscopic findings have been reported in a large series of tricuspid, bicuspid, and unicuspid aortic valves removed at surgery [2]. Overall, pathologic features became progressively more severe with valves exhibiting fewer cusps (unicuspid and bicuspid) and presented at an earlier age. For example, a decreasing number of cusps were associated with an increasing frequency of cusp calcification, calcification occurring at the raphe and the base of the cusp in the presence of ossification, cartilaginous metaplasia, and ulceration. It has also been shown that bicuspid aortic valves are more likely than tricuspid valves to show high grade (grade 4) calcification [13]. The increased severity of changes in congenital valves may result from abnormal blood flow and stress distribution across the abnormal valve causing accelerated calcification and earlier failures.
An association between congenitally malformed aortic valves and aortic dissection is well known. In one series, replacement of the ascending aorta was more common in patients with congenital valves occurring in 38.8 and 54.8 % of bicuspid and unicuspid valves, respectively, compared to 16.6 % of patients with tricuspid aortic valves [2]. Of aortic dissections reported from autopsy series, a bicuspid valve was present in 10 %, five times more than the rate of bicuspid valve in the general population. The mechanism seems to be independent of hemodynamic alterations secondary to aortic stenosis and is supported by observations that aortas associated with bicuspid aortic valves have reduced fibrillin-1 content as compared to tricuspid valves. Reduction of fibrillin-1 has been associated with release of matrix metalloproteinases that may weaken the vessel wall by degrading the elastic matrix [14].
In the most common morphology of congenital bicuspid valve (about 75 % of cases), there are two cusps of unequal size with the larger conjoint cusp located anteriorly within the aortic root; the smaller cusp is typically found posteriorly. In this morphologic variant, the right and left cusps are conjoined and both coronary arteries arise from the sinus of Valsalva from the anterior conjoint cusp. The size of the conjoint cusp is typically less than two times the size of the non-conjoint cusp and contains a median raphe (aborted third commissure). The raphe may extend a variable distance from the aortic sinus toward the cusp but usually does not reach the free edge of the conjoint cusp. Less commonly, the right and noncoronary cusps are conjoint. The rarest morphology is a conjoint left and noncoronary cusp [15].
In congenital bicuspid valves, calcification begins in the raphe (if present) and develops in a linear fashion as noted on computed tomographic examination and extends into the cusp tissue, often in a radial pattern, largely sparing the true commissures (Fig. 11.3) [11]. In contrast, in rheumatic aortic stenosis, calcification is variably present and starts in the fused commissures and extends into the body of the cusp. The bicuspid aortic valve has an uneven cusp surface that is often dysplastic in appearance. When young individuals present with symptoms of stenotic congenital bicuspid valves, the cusps are typically thickened and dysplastic. The complex anatomic features of bicuspid aortic valve including cuspal thickening and raphal ridge make the valve more susceptible to higher mechanical stress compared to the tricuspid valve. In thickened dysplastic valves, the fibrotic thickening may be diffuse or more often is most pronounced near the line of valve closure. Histologically, calcification has been reported to occur diffusely within the cusp spongiosa in congenital bicuspid valve stenosis.
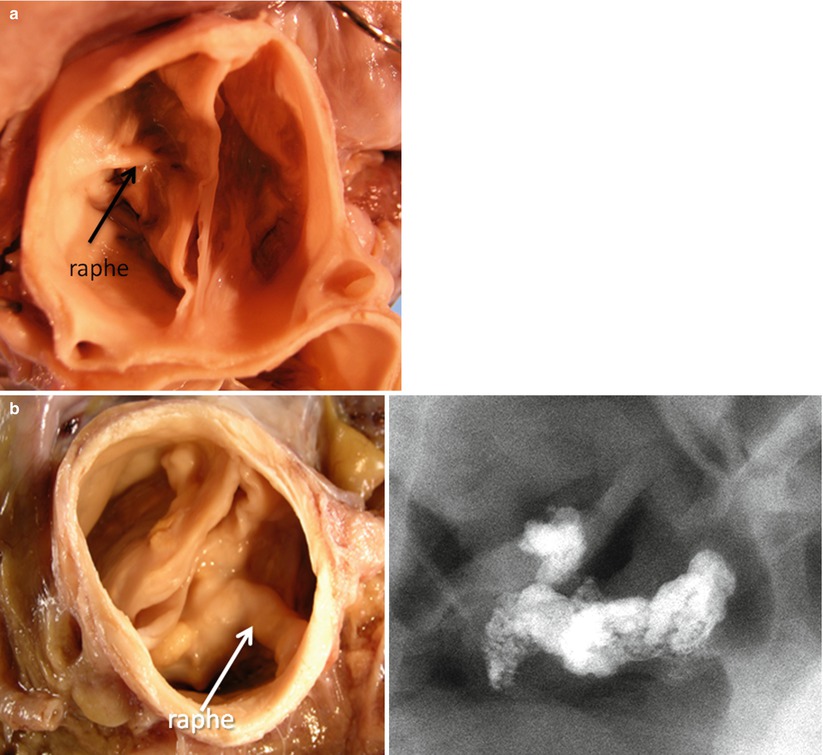
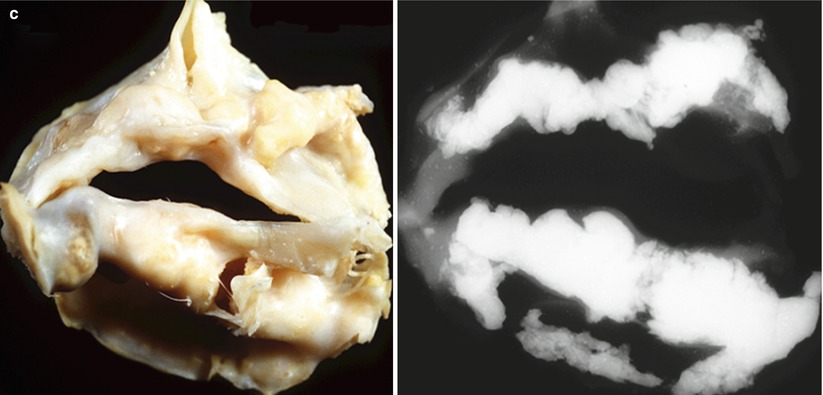


Fig. 11.3
Progression of bicuspid aortic valve calcification. (a) Bicuspid aortic valve viewed from the aortic surface (early lesion); calcification was limited to the raphe. (b) A stenotic bicuspid aortic valve obtained at autopsy from a 49-year-old male who died suddenly (late lesion). (c) Severe nodular calcification arising in a bicuspid valve. Note multiple nodules with ulceration through the aortic surface
Unicuspid valves can be categorized as one of two morphologic subtypes: (1) a domed-shaped acommissural valve containing three aborted commissures (or raphe) or (2) a unicommissural valve with slit-like opening that reaches the aortic wall at its single intact commissure. Unicommissural unicuspid aortic valves account for 60 % of aortic stenosis cases in patients <15 years old [16]. Leaflet dysplasia is common and the severity of leaflet calcification is variable however dysplasia of unicuspid has been reported to be more severe compared to bicuspid valve and is dependent on patient age [2].
Risk Factors (Box 11.1)
Several epidemiological studies have identified risk factors for calcific aortic stenosis similar to those of coronary atherosclerosis including age, male gender, smoking, elevated cholesterol levels, diabetes, metabolic syndrome, hypertension, and increased lipoprotein (a) levels. CAS has also been associated with renal dysfunction and abnormalities of calcium and phosphate metabolism such as Paget’s disease, hyperparathyroidism, and chronic renal failure [17, 18]. A recent prospective epidemiological study of 1,323 young adult participants with >25-year follow-up evaluated cardiovascular risk factors and valve calcium [19]. Of the modifiable risk factors, cigarette smoking and total cholesterol were strongly associated with valve calcium. In addition, important associations for HDL-C and BMI with valvular calcification were noted. Overall, this study provides new evidence that long-term exposure to adverse risk factors is associated with increased valve calcium [19].
Box 11.1 Risk Factors for Calcific Aortic Stenosis