- •
Coronary revascularization with CABG or PCI is a palliative intervention strategy associated with residual risks and the need for both secondary prevention lifestyle and medical therapies and recognition of the progressive nature of atherosclerotic events, which can be effectively evaluated with cardiovascular nuclear medicine imaging.
- •
SPECT and PET MPI can identify culprit ischemic territories in postrevascularization patients that benefit from the intensification of medical, lifestyle, and revascularization interventions.
- •
In postrevascularization patients with suspected ischemic symptoms or signs, exercise ECG alone without cardiac imaging is rarely preferred because the site, extent, and severity of the culprit ischemic zone cannot be assessed.
- •
Published appropriate use guidelines for SPECT and PET imaging based on current evidence and expert opinion endorsed by major cardiology and cardiac imaging societies are available to assist clinicians in the use of cardiac testing of post-revascularization patients.
- •
Current SPECT (2013) and PET (2020) appropriate use guidelines indicate MPI is appropriate more than 2 years after PCI and 5 years after CABG in patients with stable symptoms or who are asymptomatic.
- •
“Routine” annual testing with SPECT or PET MPI in patients with stable symptoms or who are asymptomatic after revascularization with PCI or CABG is rarely appropriate.
- •
Postrevascularization patients at intermediate and high risk heralded by new or recurrent ischemic symptoms, ECG changes, heart failure, or dysrhythmia are most likely to benefit from the use of SPECT and PET MPI to define therapeutic options.
Introduction
The identification of ischemia in patients after revascularization with either percutaneous coronary intervention (PCI) or coronary artery bypass graft surgery (CABG) is an important application of stress myocardial perfusion imaging (MPI). Primarily, noninvasive evaluation is indicated more for symptomatic patients with angina or dyspnea and less for screening in asymptomatic patients. Common appropriate indications for noninvasive testing in patients post-PCI or post-CABG include the development of symptoms or electrocardiographic changes consistent with ischemia, demonstration of restenosis or progression of native coronary artery disease (CAD) on coronary angiography, known incomplete revascularization or congestive heart failure, and worsening left ventricular (LV) function.
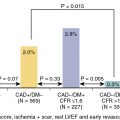
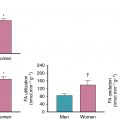
Stress imaging with radionuclide MPI, echocardiography, or cardiac magnetic resonance imaging (CMR) is preferred over stress electrocardiography (ECG) in symptomatic patients after revascularization to ascertain the location and quantify the extent and severity of ischemia in this population with previously defined angiographic coronary anatomy and prior intervention. Moreover, sensitivity for the detection of ischemia in patients after CABG because of the progression of native CAD or development of graft stenosis is much lower with stress ECG (sensitivity of ∼45%) versus stress MPI (∼68%) or stress echocardiography (∼86%) with similar specificity. In patients post-PCI, stress MPI and stress echocardiography have similar diagnostic accuracy for the detection of visual angiographic restenosis. The choice of stress MPI versus echocardiography depends on local resources and expertise. Exercise stress with imaging is preferred in patients who can exercise, and pharmacologic stress using a vasodilator or dobutamine can be employed for patients unable to exercise.
Approach to patient evaluation and test selection
Evaluating patients who have previously undergone coronary revascularization presents challenges unique to this demographic that separates them from patients with suspected CAD. Recurrent anginal symptoms may be less intense than those previously experienced before revascularization. Dyspnea may be the only presenting symptom or asymptomatic changes may be seen on routine ECGs. Nevertheless, this group represents a population at high risk for recurrent coronary artery events. As such, a lower threshold is often used by clinicians to evaluate these patients with further noninvasive testing. Although a lower threshold is employed, the published guidelines and appropriate use criteria discourage annual evaluation of such patients, particularly in the absence of new symptoms or in the asymptomatic patient. A more appropriate intervals are more than 2 years after PCI and more than 5 years after CABG and often substantially longer in the absence of new or changed symptoms, ECG repolarization changes, or dysrhythmia at rest or with exertion.
Patient preparation before MPI stress testing also requires careful consideration in the context of patients who have undergone prior coronary revascularization. If a stress MPI test is being performed to identify the burden of ischemia to consider further revascularization, then it may be appropriate to hold antianginal medication for 24 hours before the study. Alternatively, if the purpose of the study is to assess the therapeutic effectiveness of lifestyle and medical therapies in patients with known incompletely revascularized CAD, or if beta blockers are also required for control of hypertension or dysrhythmia, it may be clinically prudent to consider exercise stress testing on antiischemic medications to assess therapeutic effectiveness.
When possible, stress should be performed using exercise. Pharmacologic stress is appropriate when exercise capacity is likely to limit the diagnostic utility of the MPI stress or in the presence of left bundle branch block where vasodilator stress would be preferred. In the case of positron emission tomography (PET), although exercise stress is possible with 13 N-ammonia perfusion studies, dynamic data in such protocols for the measurement of myocardial blood flow (MBF) is not possible. It is unlikely that the additional exercise information will outweigh the potential benefits from dynamic MBF data. Thus PET MPI is likely to be performed with either vasodilator or dobutamine stress. In centers where PET and SPECT MPI are available, the advantages of PET in terms of per vessel accuracy and the additional blood flow reserve quantification obtained may encourage the use of PET over single photon emission computed tomography (SPECT). Nevertheless, by far the most commonly used MPI modality is SPECT. Despite diffuse CAD in patients with prior revascularization and the incremental diagnostic and prognostic value of quantitative flow reserve, SPECT MPI remains justifiable because relative flow quantification appears more clinically relevant and less problematic in these patients.
Patient-centered clinical applications
Effects of revascularization on myocardial perfusion and stress MPI
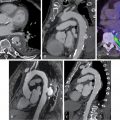
Case vignette 1: Symptomatic patient with ischemia because of “jailed” branches of the left anterior descending (LAD) artery with a patent LAD stent after PCI
A 47-year-old male with a history of hypertension, dyslipidemia, and smoking presented with an acute anterior ST-elevation myocardial infarction (STEMI). Coronary angiography demonstrated an occlusive thrombus in the proximal LAD. Subsequent successful PCI and stenting of the LAD resulted in loss or “jailing” of the septal and diagonal branches of the LAD ( Fig. 9.1 A).
Twelve months later, the patient presented with symptoms consistent with stable angina (Classes I and II) and a limited exercise capacity. A rest and dipyridamole-stress 99m technetium (Tc)-tetrofosmin SPECT MPI study was performed. The dipyridamole-stress ECG was negative for ST depression and no angina occurred during stress. The stress images demonstrated a moderate to severe reduction of tracer uptake in the basal to mid segments of the septal wall and mild to moderate reduction in the basal anterior and basal anterolateral segments (see Fig. 9.1 B). On rest imaging, there was mild improvement in tracer uptake in both regions consistent with mild ischemia and nontransmural scarring in the septal and diagonal branch territories of the LAD and a patent LAD stent. The images were congruent with the angiographic findings after stenting. The SPECT images also demonstrated an inferior attenuation artifact that was improved with prone imaging.
Case vignette 2: Symptomatic patient with ischemia due to nonrevascularized vessel segments after CABG (serial LAD lesions with patent LAD graft and septal and diagonal ischemia)
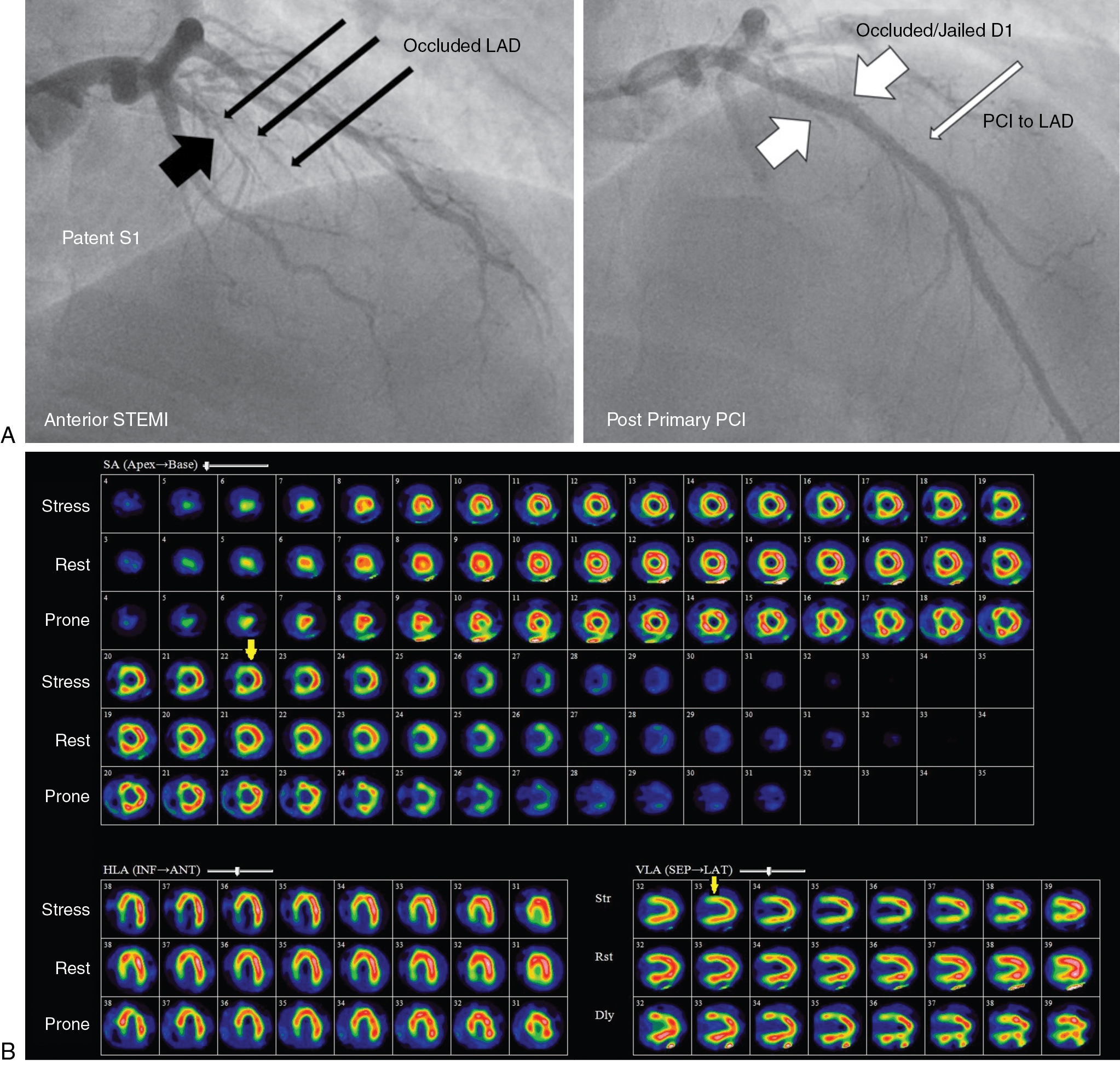
A 62-year-old female with a history of diabetes mellitus and dyslipidemia returned for a routine annual assessment with symptoms of chest pain on moderate exertion (Class II). She had had a non-STEMI and CABG 2 years before the current presentation. Coronary angiography had revealed a severe proximal LAD stenosis and a 100% occlusion of the mid LAD without significant stenoses in the left circumflex (LCx) or dominant right coronary (RCA) arteries ( Fig. 9.2 A). The mid and distal LAD filled late via faint collaterals from the RCA contrast injection. The patient underwent CABG with a left internal thoracic artery (LITA) graft to the distal LAD and right internal thoracic artery (RITA) graft to the first diagonal branch of the LAD.
The patient underwent a rest and dipyridamole-stress 82 Rb PET study without ST depression or angina. On the stress perfusion images (see Fig. 9.2 B), there was a small perfusion defect of severe intensity involving the mid and basal septal segments showing complete reversibility at rest, consistent with moderate ischemia in the distribution of the septal branches of the LAD. A small, fixed apical partial volume artifact was also seen on rest and stress images. Otherwise, perfusion of the LAD territory was normal, suggesting patency of the LITA and RITA grafts with good flow (see Fig. 9.2 C). Flow quantification analysis (see Fig. 9.2 D) showed moderately reduced stress flow in the septal region and mildly reduced flow in most of the LAD territory, possibly due to mild diffuse epicardial CAD. Rest flow was near normal. Myocardial flow reserve (MFR) was reduced in the septal region. Global MFR was normal (>2), suggesting a good prognosis. Attention to optimizing risk factor management and medical management of angina was advised.
Case Vignettes 1 and 2 illustrate how the preexistence of disease in revascularized patients influences the technical approach to image analysis. Imaging of ischemia in patients after revascularization is more complicated than in patients with only native vessel CAD. Ischemia may result from in-stent restenosis or graft disease, incomplete revascularization, or progression of CAD in treated or untreated coronary arteries. Moreover, patients after PCI with patent stents may have ischemia because of jailed branches, such as septal perforators or diagonal branches with LAD stenting. These patients with patent stents often have typical MPI patterns that can be identified and suggest stent patency, possibly avoiding unnecessary invasive coronary angiography. The MPI pattern can be compared with the pre- and postprocedural coronary angiograms in the post-PCI patient to identify jailed branches, which are usually not amenable to stenting (see Case Vignette 1). Similarly, patients after CABG may have ischemia with patent grafts but poor retrograde flow to branches in a grafted artery because of severe sequential stenoses or a proximal stenosis. This may produce an unusual pattern of proximal ischemia with relatively normal distal myocardial perfusion that would be hard to explain anatomically without prior knowledge of distal vessel grafting (see Case Vignette 2).
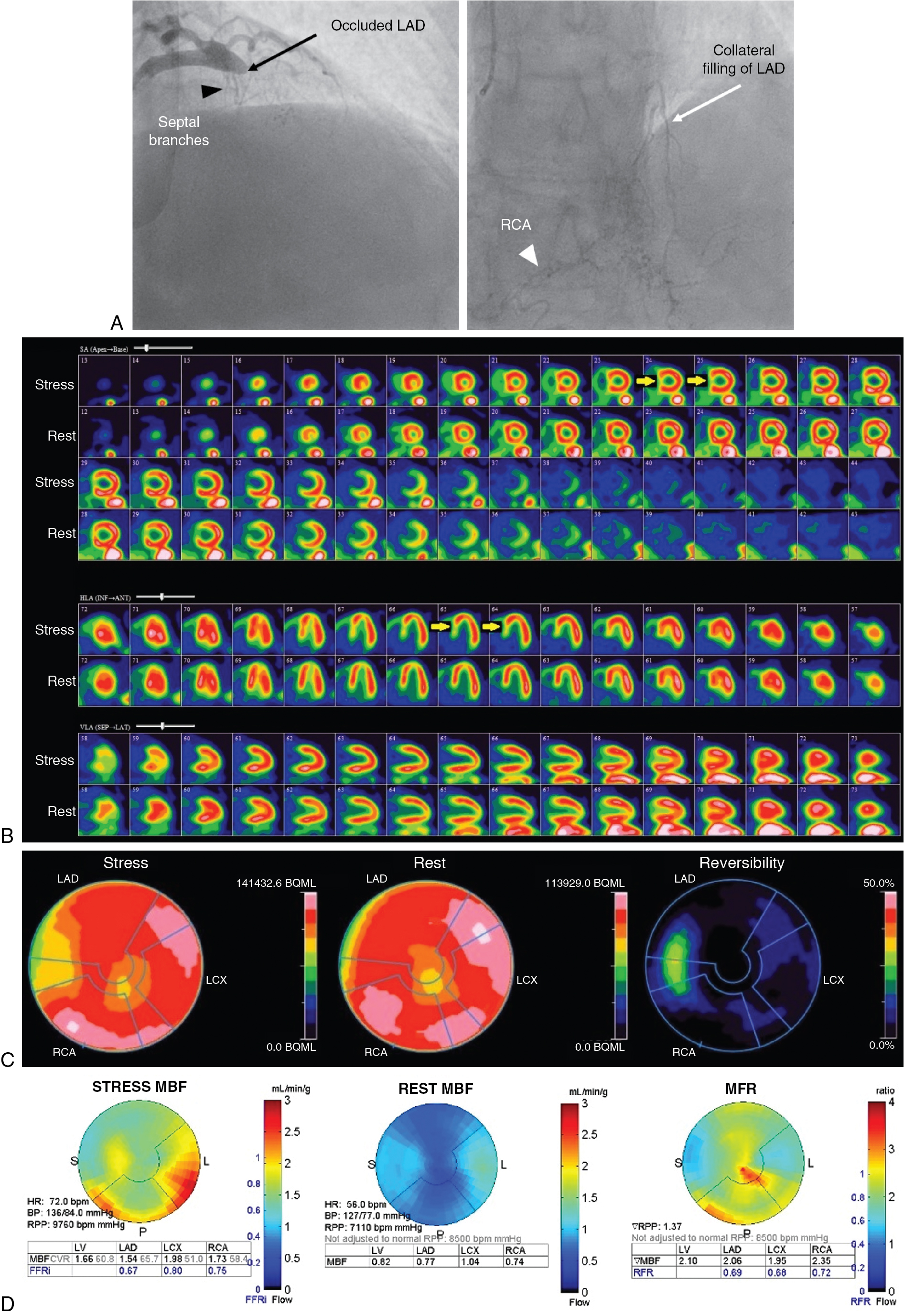
Case vignette 3: Symptomatic patient managed with CABG and subsequent improvement in stress myocardial flow
A 70-year-old female with dyslipidemia and hypertension presented with exertional angina (Class II). The patient underwent a rest and dipyridamole-stress 82 Rb PET MPI ( Fig. 9.3 A). The stress images demonstrated a large perfusion defect of severe intensity throughout the mid and apical anterior and anteroseptal walls, apical LV segments, and LV apex, showing complete reversibility, consistent with severe ischemia in the mid LAD territory. The quantitative MBF polar maps showed severely reduced stress flow and flow reserve in the LAD territory and relatively preserved flow in the LCx and RCA territories. Coronary angiography revealed multiple heavily calcified severe stenoses of the mid LAD without obstructive stenosis in the LCx or RCA. A LITA to the distal LAD was successfully performed.
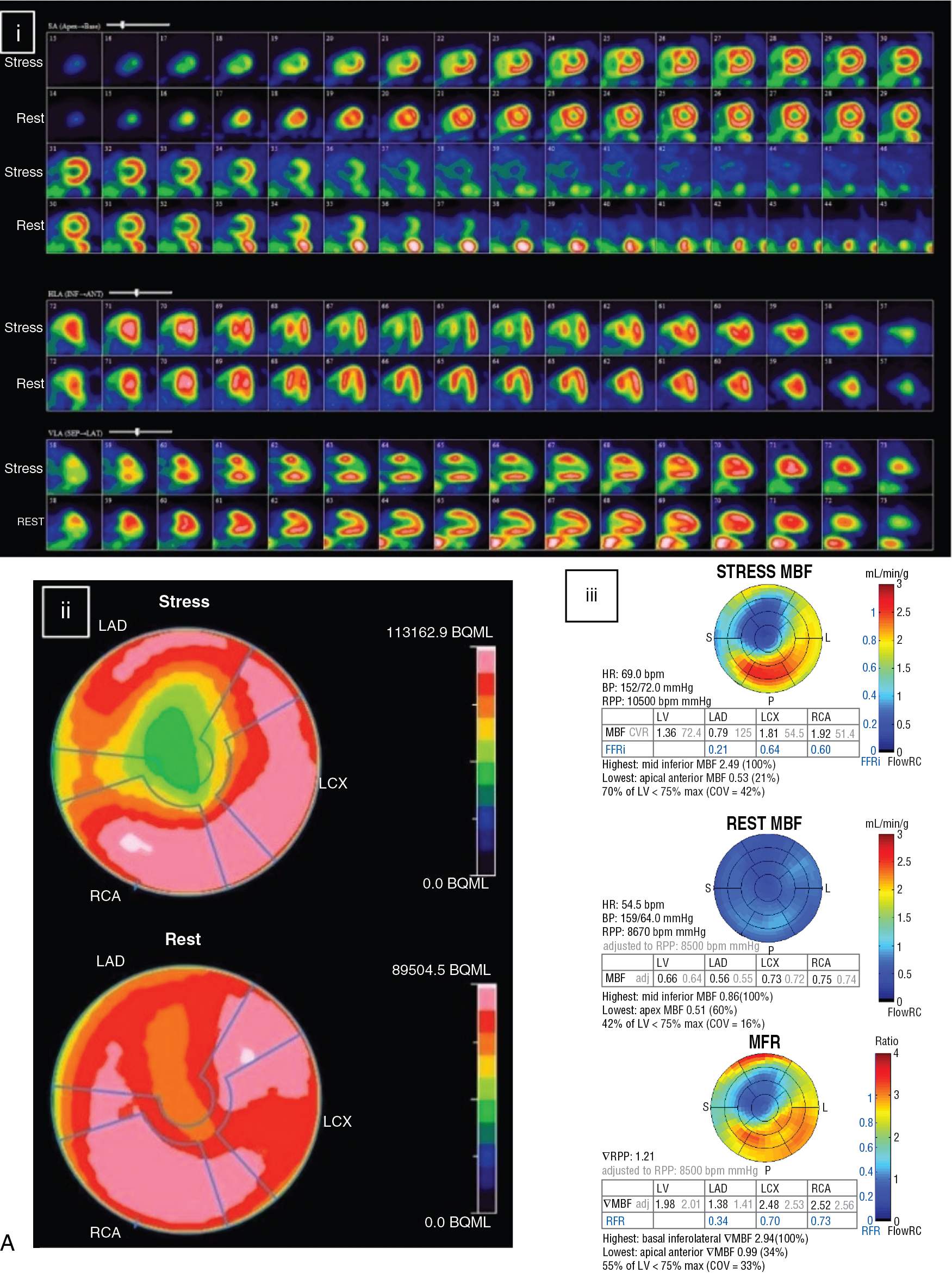

The patient returned 12 months after CABG for routine cardiology assessment and complained of atypical angina. A repeat rest and dipyridamole-stress 82 Rb PET MPI was performed (see Fig. 9.3 B). The post-CABG 82 Rb PET images showed normal myocardial perfusion on the rest and stress images. MBF quantification was much improved and the stress MBF and normal MFR in the LAD territory were nearly normal. The patient was reassured about the patency of the LITA and the absence of significant progression of atherosclerosis in her ungrafted vessels.
Stress MPI in a patient after PCI or CABG is frequently normal and usually demonstrates much improvement in relative perfusion with SPECT or PET and absolute rest and stress MBF and MFR with PET. These results can be very reassuring in a symptomatic patient, help exclude an ischemic etiology for the patient’s chest pain, and predict a good prognosis.
Evaluation of symptomatic patients after revascularization with stress MPI to localize ischemia
Case vignette 4: Symptomatic patient after PCI evaluated with exercise SPECT MPI to a jailed diagonal branch of the LAD
A 51-year-old male presented with chest discomfort, which he described as epigastric and retrosternal burning brought on by meals and strenuous exertion. He stated it started 4 weeks earlier as part of a weight loss program. Vascular risk factors included dyslipidemia, hypertension, and a family history of premature CAD. History included PCI 1 year ago with stenting of proximal 70% and mid 100% LAD lesions and no other obstructive coronary lesions. Rest ECG was normal.
The patient was referred for a rest and exercise-stress 99m Tc-tetrofosmin SPECT MPI. The patient exercised for 9 minutes and 34 seconds with a peak heart rate of 150 beats per minute (88% of age-predicted maximal heart rate), normal blood pressure response, and no ST-segment depression or angina. The patient stopped because of fatigue and achieved a work level of 11.9 metabolic equivalent tasks (METS). The Duke Treadmill score was calculated as 9 (low risk). The perfusion images showed a small area of mild ischemia in the basal anterolateral wall consistent with a jailed diagonal branch of the LAD (% LV ischemia of 1.5%) and were otherwise normal ( Fig. 9.4 ). ECG-gated images showed normal ejection fraction, size, and wall motion at rest and after exercise. The very small amount of ischemia observed on the perfusion images was probably related to the PCI procedure. The patient was reassured that the LAD stent was most likely patent and that he had a good prognosis without the need for further investigations, such as invasive coronary angiography.