chapter 14 Pediatric Tumors
INTRODUCTION
Approximately 8600 new cases of childhood cancer are diagnosed each year in the United States.1 In addition, there are an estimated 1600 deaths in children secondary to malignancy. Leukemias, lymphomas, and central nervous system tumors account for 55% to 60% of all childhood cancer, with soft tissue and bone, sympathetic system, and kidney tumors comprising another 35%. Carcinomas, the most frequent group of cancers in adults, are infrequent in children. Unlike in adults, there is less information on the use of positron emission tomography (PET) or positron emission tomography-computed tomography (PET-CT) in the diagnosis, treatment planning, and follow-up of patients with childhood tumors. Most of the reported literature on PET or PET-CT in the management of pediatric malignancies has been in lymphomas and bone or soft tissue sarcomas.
Special Issues in Children
Children have different issues regarding the use of PET-CT when compared to adults. A reliable intravenous access is important in patient preparation because children and their parents generally do not tolerate multiple access attempts. Bladder catheterization may be needed to avoid obscuring lesions by reconstruction artifacts in the pelvis and the possibility of spontaneous voiding during image acquisition and resultant contamination.2 Children may need anesthesia for a PET-CT scan just as they do for other procedures in pediatric radiology.
Radiation doses for pediatric patients should be kept as low as reasonably achievable. Administered doses of 18F-fluoro-deoxy-D-glucose (FDG) ranging from 5 to 10 MBq (0.15–0.30 mCi)/kg have been recommended for use in children.3 The higher radiation exposure of PET-CT in comparison to PET as a single modality has been a concern because of a possible higher risk of radiation-induced malignancies.4 The use of a low-dose CT (80 kVp, 10 mA, 0.5 s per rotation) with a radiation dose (0.3 mGy) that is 100 times less than necessary for a diagnostic-quality CT has been recommended for anatomic correlation.5 However, even low-dose CT attenuation correction is associated with radiation exposure for the child nearly 10 times that of radioactive source-based attenuation correction (0.035 mGy). FDG uptake has also been shown to be higher in infants and children than in adults with respect to the bladder, brain, heart, liver, and pancreas.2
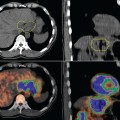
The interpretation of PET-CT images in children can also be different when compared to those in adults. The calculation of the standardized uptake value (SUV) in pediatric patients based on body surface area seems to be more of a uniform parameter than an SUV calculation based on body weight.6 Intense FDG activity can be found in the adenoids, tonsils, supraclavicular or brown fat, as well as muscle bundles. Increased uptake in the adenoids or tonsils can be difficult to interpret, especially in children with lymphomas, where this site can be affected by tumor. In addition, the thymus often shows mild to moderate diffuse FDG uptake after chemotherapy, which is secondary to a rebound phenomenon. Likewise, the bone marrow after chemotherapy in children may show diffuse intense uptake due to increased cell proliferation after myelosuppresion. Flat and symmetrical areas of mild FDG uptake can occur in the epiphyseal cartilage secondary to hypermetabolic activity.
Rationale for Use of PET or PET-CT in Pediatric Tumors
Hodgkin’s Disease and Non-Hodgkin’s Lymphomaz
Hodgkin’s disease makes up 6% of all childhood cancers. Most present with a pattern of contiguous lymphatic spread. Cervical adenopathy is seen in 80% of patients, while mediastinal involvement occurs in 50%. Splenic involvement can occur, while extranodal involvement is not common. Diagnostic imaging includes CT of the neck, chest, abdomen, and pelvis. Nuclear imaging with gallium-67 is widely used to stage and monitor response to treatment. Gallium-67 persistence in the mediastinum after completion of therapy has been shown to correlate with future relapse.7 Gallium-67 is limited in its ability to evaluate abdominal and pelvic lymph nodes because of its low resolution and physical biodistribution.8
Recently FDG-PET has been used for staging and surveillance after treatment. In fact, PET has gradually replaced 67Ga as the preferred functional imaging test for lymphomas.9,10 FDG-PET has advantages over 67Ga because the scan is a 1-day procedure with higher resolution, better dosimetry, and less intestinal activity.8 On the other hand, 67Ga requires delayed imaging over a period of 72 hours, which is inconvenient and not cost-effective.
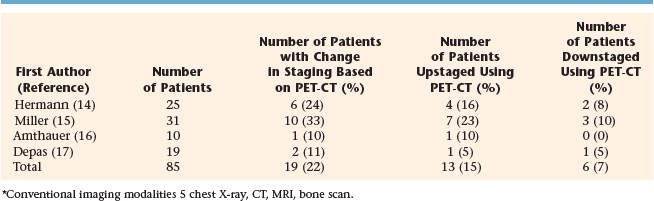
PET has been compared to CT, magnetic resonance imaging (MRI), and PET-CT in the interpretation of sites of disease in children with Hodgkin’s and non-Hodgkin’s lymphoma.11–13 In a study from Berlin, four methods of imaging were compared with regard to accuracy in detecting childhood Hodgkin’s disease: conventional imaging modality or CIM (CT of the thorax and MRI of the neck, abdomen, and pelvis), PET, side-by-side analysis (SBS), of PET, and CIM and PET-CT fusion. For nodal regions the accuracy rates for CIM, PET, SBS, and PET-CT were found to be 86%, 89%, 94%, and 97%, respectively. For extranodal regions, the rates were 96%, 96%, 100%, and 100%, respectively. Staging and treatment were correctly modified by SBS in five and four patients, and by PET-CT fusion in seven and five patients.11 A study of 18 children with Hodgkin’s disease and six with non-Hodgkin’s lymphoma identified 414 regions of which both PET and CT were concordant in 366 (positive in 16 and negative in 350). Discordance was found in 48 regions. Overall sensitivities, specificities, and positive and negative predictive values were (respectively) 78%, 98%, 94%, and 90% for FDG-PET and (respectively) 79%, 88%, 90%, and 46% for CT scan.12 Table 14-1 shows the number of patients whose initial stage was altered by the use of PET-CT in comparison to more conventional imaging modalities.14–17 Overall, approximately 22% of patients with lymphoma had a change in stage with PET-CT; a total of 15% were upstaged while 7% were downstaged.
TABLE 14-1 Comparison of PET-CT and Conventional Imaging Modalities* in the Initial Staging of Childhood Lymphoma
The use of FDG-PET in surveillance after treatment for childhood lymphoma is still under investigation. Two studies have shown that a negative PET-CT after treatment strongly suggests absence of disease.15,18 A positive PET-CT, however, should be interpreted with caution. In one study, the positive predictive value of PET during follow-up was only 11% with a false-positive rate of 16%.19 In another study, the positive predictive value of a PET scan was 53%.18
Bone Tumors
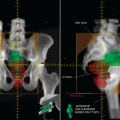
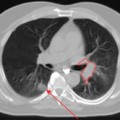
Ewing’s sarcoma accounts for 3% of all pediatric malignancies and is the second most common childhood primary bone cancer. Treatment includes multi-agent chemotherapy with surgery and/or radiotherapy for local control. Most present with localized disease, but 25% have distant metastasis at initial diagnosis. The most common sites of distant spread are the lungs, bone, and bone marrow. Standard radiologic workup includes a CT scan and MRI of the primary site to determine local tumor extent, CT scan of the chest, abdomen and pelvis, and a bone scan. Recently, there have been published reports of the use of PET or PET-CT in the initial diagnosis and follow-up of patients treated for Ewing’s sarcoma.20–25 In one study, the addition of PET to standard workup upstaged 3 of 17 patients (18%); this led to alteration of treatment by substitution of radiation therapy in lieu of surgery for local control in Ewing’s sarcoma.23 In another study from Freiburg, the sensitivity and specificity for detection of the primary tumor and metastatic lesions were 96% and 78%, respectively. In four patients with bone metastasis, 70 lesions were detected by PET while eight were detected by bone scan. The authors concluded that PET is better than bone scan in detection of bone metastases.20 Response to neoadjuvant chemotherapy can also be detected using PET. A series of 36 patients treated in Seattle showed that the SUV after initial chemotherapy and just prior to surgery was predictive of progression-free survival. For SUV < 2.5, the 4-year progression-free survival was 72% while for SUV 2.5, it was 27%.21
Osteosarcoma is the most common primary malignant bone tumor in children. Treatment usually consists of multi-agent chemotherapy and surgery for local control. Because of its inherent radio-resistance, radiation therapy is often not used for local control except in cases of unresectability. The most common sites of distant metastases are the lung and the bone. Like Ewing’s sarcoma, the standard radiologic tests to determine tumor extent and spread include a CT and MRI of the primary site, CT of the chest, and bone scan. PET has also recently been used in the staging and follow-up of children and adolescents with osteosarcoma.23,24,26 A study from North Carolina showed that 1 of 38 patients (3%) was upstaged by the addition of PET to conventional imaging.23 With regard to evaluation of neoadjuvant chemotherapy response by FDG-PET in osteosarcoma, there have been conflicting results, with one study showing that FDG-PET is a promising tool for correlation of degree of tumor necrosis while another showed the opposite result.27,28 Franzius and colleagues showed that recurrences from osteosarcoma and Ewing’s sarcoma are more readily identified by PET when compared to conventional studies. In a study of 27 patients, the sensitivity, specificity, and accuracy for detection of recurrences by PET were 96%, 81%, and 90%, respectively, while for detection by conventional imaging, the corresponding rates were 100%, 56%, and 82%, respectively.26
Rhabdomyosarcoma and Other Soft Tissue Sarcoma
FDG-PET has recently been used in the initial staging and detection of recurrence from pediatric soft tissue sarcoma.29–32 Most of the literature involves rhabdomyosarcoma. Investigators from Memorial Sloan-Kettering Cancer Center found that PET had 77% sensitivity and 95% specificity in detection of rhabdomyosarcoma in the primary and metastatic sites.29 All primary sites were detected by FDG-PET. In another study, three patients with alveolar rhabdomyosarcoma of the extremity had PET-positive axillary nodes; two were found to have nodal involvement while one was pathologically negative.30 In a study of four patients, a decrease in FDG uptake was correlated with favorable response to chemotherapy while a persistent abnormality in FDG uptake was found in the patient who later progressed.31 For detection of recurrence, FDG-PET correctly identified local recurrence in six of six cases and distant metastasis in four of six.32
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree