Pediatrics
Hossein Jadvar
Leonard P. Connolly
Frederic H. Fahey
Barry L. Shulkin
The use of positron emission tomography (PET) and the more recent hybrid PET with computed tomography (PET/CT) systems is rapidly expanding. The development and validation of applications for PET in pediatrics has proceeded more slowly than in adult medicine, but PET and PET/CT are becoming important in the diagnostic imaging evaluation of children with a variety of disorders, particularly cancer.
The initial slow proliferation of PET in pediatrics was partly because diseases to which PET has been most widely applied in adults are uncommon in pediatrics. Only about 2% of all cancers, for example, occur in patients <15 years of age. Experience has therefore been gained more slowly in pediatrics. Accrual of experience has been further slowed since there are few PET units in pediatric hospitals.
The labor-intensive nature of imaging sick children has limited the ability of adult-oriented PET centers, which are faced with a shortage of available imaging slots, to take on a substantial number of time-consuming, challenging pediatric cases. Despite these considerations, PET and PET/CT are emerging as important tools in pediatric nuclear medicine. This chapter will review the clinical applications of PET and PET/CT in pediatrics with an emphasis on the more common applications in epilepsy and oncology. General considerations in patient preparation and radiation dosimetry will also be discussed.
Patient Preparation
Preparation of children and parents for nuclear medicine imaging has been thoroughly reviewed elsewhere (1,2). As with any imaging study, gaining the trust and allaying the fears of both the patient and the parents are essential before attempting to image. Because parental attitudes and anxieties are readily conveyed to children, it is essential that the parents be well informed and cooperative if their child’s trust is to be gained. Patient cooperation, once achieved, may be assisted by relatively simple methods. Sheets wrapped around the body, sandbags, and/or special holding devices are often sufficient for immobilization. Parents may accompany their child during the course of the study to provide emotional support. However, on occasion, an anxious parent may impair the performance of the study.
Sedation is indicated when, on the basis of careful consideration, it is anticipated that simpler approaches will be inadequate. Sedation protocols, particularly regarding the recommended medications and the level of sedation required for an imaging procedure, vary from institution to institution. Guidelines such as those advanced by the Society of Nuclear Medicine are useful in developing an institutional sedation program and a sedation formulary (3). Also important to consider is the potential effect of sedatives on fluorodeoxyglucose (FDG) distribution. Many sedatives may affect cerebral metabolism. When performing FDG PET of the brain, it is best if sedatives are not administered for 30 minutes after FDG administration because it is during this interval that the majority of cerebral FDG uptake occurs. Sedatives are not known to cause significant changes in tumoral metabolism and can be administered at any time relative to FDG administration for studies of tumors outside the central nervous system (4). It should be noted that the effects of sedation on FDG uptake in tumors have not been exhaustively studied, however, and it is suggested that a similar approach to brain PET imaging might be considered, with sedation starting after the FDG uptake has been substantial, such as after 30 minutes post tracer injection.
Other important technical issues specific for the performance of PET studies in pediatric patients (e.g., consent, intravenous access, bladder catheterization) have been reviewed (5,6). The recent introduction of PET/CT imaging systems also presents unique issues that will need to be addressed. Kaste (7) has reviewed the experience with implementing PET/CT at a tertiary pediatric hospital. Issues such as physical location of the PET/CT unit, the roles of CT and nuclear medicine technologists, and the methodology for study interpretation are discussed. Additional important considerations deliberated are the use of intravenous and sugar-free oral contrasts for the CT portion of the PET/CT examinations and the management of hyperglycemia. Procedure guidelines for tumor imaging with PET and PET/CT have been published (8,9).
Table 8.23.1 Radiation Dosimetry for Fluorodeoxyglucose | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Radiation Dosimetry
Several factors affect the dosimetry of positron emitters relative to single photon imaging agents. On the one hand, the energy per photon is higher (511 keV as compared to 140 keV for technetium-99m [99mTc]), and there are two photons emitted per disintegration. This leads to higher energy per unit activity than with most single photon agents. However, the higher photon energy also leads to a smaller fraction of the photons being absorbed within the patient. Table 8.23.1 summarizes the dosimetry of FDG for selected organs as well as the effective dose in the pediatric population based on the administered activity of 5.55 kBq/kg (0.15 mCi/kg).
Since the administered activities are scaled by body weight, the doses are similar across the age range being slightly higher in adults. The effective dose is 5.1 and 7.4 mSv for the 1-year-old and the adult, respectively. The critical organ is the bladder wall with the dose being six to eight times higher than the effective dose (based on a 2-hour voiding; however, patients routinely void before image acquisition starts about 1-hour postinjection). Table 8.23.2 compares the effective dose from FDG to a number of commonly used, single photon imaging agents. From this table, it can be seen that the radiation absorbed dose to the patient from an FDG PET scan is very similar to the dose received from other nuclear medicine imaging procedures (10,11).
Table 8.23.2 Effective Dose in Pediatrics for a Variety of Radiopharmaceuticals | |||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||
In many cases in pediatric imaging, the parents of the patient prefer to remain with the patient during the procedure. The exposure rate constants for fluorine-18 ([18F]) and 99mTc are 0.0154 and 0.00195 mR/h per MBq at 1 m, respectively. The difference is primarily due to the higher photon energy for [18F] as compared to 99mTc, and the fact that two photons are emitted per disintegration. It is also therefore prudent to consider the radiation exposure to the parent during these procedures. As shown in Table 8.23.1, pediatric patients receive a range of administered activities depending on patient size. Consider the following assumptions: the patient receives 260 MBq and is considered to be a point source with no self-absorption. The patient sits in a preparatory room for 60 minutes during uptake and then is imaged for 60 minutes. These assumptions are considered quite conservative; that is, these will probably lead to an overestimation of the radiation dose to the parent. Table 8.23.3 estimates the total exposure to the parent during both the uptake and imaging periods, provided the parent maintains the distance from the patient specified.
Even if the parent stayed within 1 m of the patient during the entire uptake and imaging periods, the exposure to the parent
would be no more than 5.5 mR. Therefore, the parents can be allowed to stay with the patient during the procedure but are instructed to stay as far from the patient as they feel comfortable. Hybrid PET/CT scanners use the CT portion of the examination for attenuation correction. The dose to the patient from CT can vary greatly depending on the tube voltage and current and the size of the patient. Table 8.23.4 summarizes the dose to patients of various ages (based on a phantom study using phantoms of various sizes) as a function of tube voltage.
would be no more than 5.5 mR. Therefore, the parents can be allowed to stay with the patient during the procedure but are instructed to stay as far from the patient as they feel comfortable. Hybrid PET/CT scanners use the CT portion of the examination for attenuation correction. The dose to the patient from CT can vary greatly depending on the tube voltage and current and the size of the patient. Table 8.23.4 summarizes the dose to patients of various ages (based on a phantom study using phantoms of various sizes) as a function of tube voltage.
Table 8.23.3 Total Exposure to The Parent from a Patient Receiving 260 MBq of Fluorine-18 for a Fluorodeoxyglucose PET Study | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||
Smaller patients receive a substantially higher dose from the same CT acquisition parameters. For example, a 10-year-old will receive approximately twice the radiation dose of a medium-sized adult for the same CT acquisition parameters. An alternative to using CT for attenuation correction is to use rotating rod sources. Based on a phantom study using phantoms of different sizes, the dose to the patient is between 0.05 and 0.2 mGy for 15 minutes of scanning with a total activity in the rods of 370 MBq. Thus the dose to the patient from a CT scan used for attenuation correction is substantially higher than that associated with the rotating rods’ sources. However, the CT scan provides anatomical correlation to the functional images, a feature that is not available using the rod sources and is considerably quicker, which can be of particular value for pediatric imaging.
Comparing the values in Tables 8.23.1 and 8.23.4, the dose to the patient from the CT portion of the scan can be equal to, if not higher than, the dose received from the radiopharmaceutical. The acquisition parameters for the CT portion of the scan should be tailored to the patient’s size. For diagnostic CT, reduction of exposure by 30% to 50% relative to adult has been suggested (12). Reducing the milliamperes proportionately decreases the absorbed radiation dose without significant loss in the information provided. In addition, there is the potential to further reduce the tube voltage and current without adversely affecting the quality of the attenuation correction in those cases where precise anatomical correlation is less important.
Table 8.23.4 Dose from CT at Different Ages | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||
PET in Pediatric Neurology
A discussion of brain PET imaging in all age groups is present in Chapter 9.1. In the following sections the PET imaging in neurologic applications is discussed.
Normal Brain Development
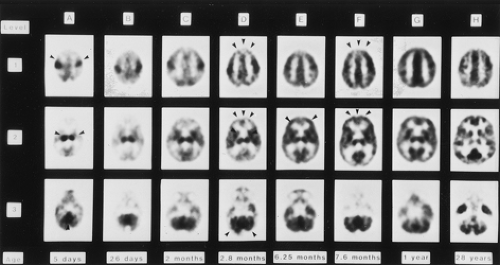
An understanding of the normal brain development and evolution of cerebral glucose utilization is important when FDG PET is considered as a diagnostic functional imaging study. Functional maturation proceeds phylogenetically. Glucose metabolism is initially high in the sensorimotor cortex, thalamus, brainstem, and cerebellar vermis. During the first 3 months of life, glucose metabolism gradually increases in the basal ganglia and in the parietal, temporal, calcarine, and cerebellar cortices. Maturation of the frontal cortex, which proceeds from lateral to medial, and the dorsolateral cortex occurs during the second 6 months of life. Cerebral FDG distribution in children after the age of 1 year resembles that of adults (Fig. 8.23.1) (13,14,15).
Epilepsy in Childhood
Epilepsy is a relatively common and potentially devastating neurologic condition during childhood. Its incidence in children and adolescents is between 40 and 100 per 100,000 (16). The 1990 National Institutes of Health Consensus Conference on Surgery for Epilepsy estimated that 10% to 20% of epilepsy cases prove medically
intractable, and that 2,000 to 5,000 epilepsy patients per year can benefit from surgical resection of the seizure focus (17). Accurate preoperative localization of the epileptogenic region is an essential but difficult task that is best accomplished by finding a concordance between results obtained with clinical examination, electroencephalography (EEG), neuropsychological evaluation, and imaging studies. CT and magnetic resonance imaging (MRI) are used to detect anatomic lesions that may cause the seizures. However, structural lesions occur in a relatively small percentage of patients with epilepsy, and when such lesions are detected, they may not necessarily correspond to the epileptogenic region (18). Ictal or interictal single-photon emission tomography (SPECT) evaluation of regional cerebral blood flow (rCBF) with tracers such as 99mTc-hexamethylpropylene (99mTc-HMPAO) and 99mTc-ethylcysteinate dimer (99mTc-ECD) can localize the epileptogenic region in the presence or absence of structural abnormalities. The characteristic appearance of an epileptogenic region is relative zonal hyperperfusion on ictal SPECT and relative zonal hypoperfusion on interictal SPECT.
intractable, and that 2,000 to 5,000 epilepsy patients per year can benefit from surgical resection of the seizure focus (17). Accurate preoperative localization of the epileptogenic region is an essential but difficult task that is best accomplished by finding a concordance between results obtained with clinical examination, electroencephalography (EEG), neuropsychological evaluation, and imaging studies. CT and magnetic resonance imaging (MRI) are used to detect anatomic lesions that may cause the seizures. However, structural lesions occur in a relatively small percentage of patients with epilepsy, and when such lesions are detected, they may not necessarily correspond to the epileptogenic region (18). Ictal or interictal single-photon emission tomography (SPECT) evaluation of regional cerebral blood flow (rCBF) with tracers such as 99mTc-hexamethylpropylene (99mTc-HMPAO) and 99mTc-ethylcysteinate dimer (99mTc-ECD) can localize the epileptogenic region in the presence or absence of structural abnormalities. The characteristic appearance of an epileptogenic region is relative zonal hyperperfusion on ictal SPECT and relative zonal hypoperfusion on interictal SPECT.
The sensitivity of ictal rCBF SPECT may approach 90%, while that of interictal SPECT is in the range of 50% (19). The utility of ictal SPECT is somewhat reduced by the difficulty in coordinating tracer administration with seizures. Noninvasive evaluation is often unsuccessful in precisely localizing an epileptogenic region. As a result, surgical placement of electrode grids on the brain surface or insertion of depth electrodes becomes necessary.
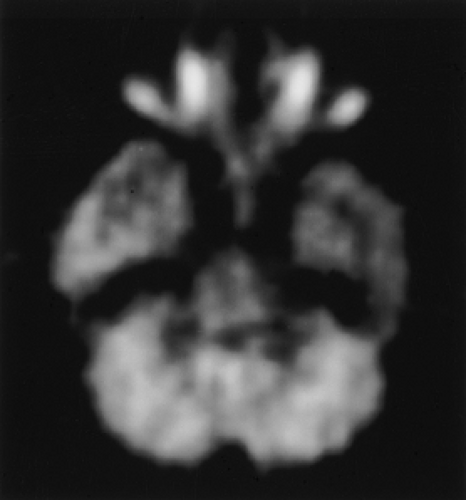
FDG PET has proven useful in preoperative localization of the epileptogenic region (Fig. 8.23.2) (20). FDG PET is generally performed following an interictal injection. Although metabolic alterations might be localized better ictally than interictally, the relatively short half-life of [18F] limits the window of opportunity during which it can be administered ictally. Even when FDG can be administered at seizure onset, the approximately 30-minute brain uptake time of FDG means that the study may depict peri-ictal as well as ictal FDG distribution. Ictal FDG studies may also show areas of seizure propagation, which could be confused with the actual seizure focus. Despite these considerations, some favorable results have been reported employing ictal PET for patients with continuous or frequent seizures (21).
For interictal PET, FDG should be administered in a setting such as a quiet room with dim lights where environmental stimuli are minimal during the 30 minutes following FDG administration. It is best to have the child remain awake with minimal parental interaction during this period. EEG monitoring is essential to identify seizure activity that might affect FDG distribution.
The sensitivity of interictal FDG PET approaches that of ictal rCBF SPECT in localizing the epileptogenic region, which is detected as regional hypometabolism (22). Importantly, the hypometabolism may predominantly affect cortex bordering the epileptogenic focus. Epileptic activity may originate in cortical areas bordering the hypometabolic regions rather than the hypometabolic region itself (23). A recent study also suggested that persistent or increased seizure frequency (one or more seizures per day) results in enlargement in the area of hypometabolic cortex on sequential PET scans. In contrast, patients whose seizure frequency decreases below daily seizures show a decrease in the size of the hypometabolic cortex on serial scans (24).
Incorporation of FDG PET into preoperative evaluation of patients with epilepsy reduces the need for intracranial EEG monitoring and the cost of preoperative evaluation (25,26). The best results have been obtained in temporal lobe epilepsy, for which metabolic abnormalities may be evident in as many as 90% of surgical candidates (25,27). Coregistered FDG PET and MRI in association with diffusion tensor imaging has also been recently shown to accurately localize the epileptogenic cortex in patients with tuberous sclerosis complex (28).
Extratemporal epileptogenic regions are more difficult to identify, but some success has been achieved in children with intractable frontal lobe epilepsy and normal CT or MRI studies (29). FDG PET has been reported as being of particular help in the evaluation of infantile spasms, a subtype of seizure disorder. This condition, which has an incidence of 2 to 6 per 10,000 live births, consists of a characteristic pattern of infantile myoclonic seizures and is frequently associated with profound developmental delay despite medical treatment (15,30). Prior to the availability of FDG PET, surgical intervention was attempted and successful in only isolated instances. Incorporation of FDG PET into the evaluation of children with infantile spasms has resulted in identification of a substantial number of children who have benefited from cortical resection. FDG PET has revealed marked focal cortical glucose hypometabolism associated with malformative or dysplastic lesions that are not evident on anatomic imaging. There is a marked decline in seizure frequency and in some patients reversal of developmental delay when a single metabolic abnormality that correlates with EEG findings is shown by FDG PET. Patients with bitemporal hypometabolism on FDG PET have a poor prognosis and typically are not candidates for resective surgery (31,32,33,34).
In addition to FDG, PET tracers that assess an altered abundance or function of receptors, enzymes, and neurotransmitters in epileptogenic regions have been applied to localizing the epileptogenic region. Among alterations that have been observed are relatively reduced uptake of carbon-11 ([11C])-flumazenil, a central benzodiazepine receptor antagonist, and [11C]-labeled (S)- [N-methyl] ketamine, which binds to the N-methyl-D-aspartate receptor-gated ion channel, and the amino acid methyl-Ltryptophan (35,36,37,38,39,40,41). Relative increases in uptake of [11C]-carfentanil, a selective mu opiate receptor agonist, and [11C]-deuterium-deprenyl, an irreversible inhibitor of monoamine oxidase type B, have also been described (42,43).
Other Neurologic Applications
PET with oxygen-15 ([15O])-water has also been investigated in infants with intraventricular hemorrhage and hemorrhagic infarction and in infants with hypoxic ischemic encephalopathy (44,45). Cerebral blood flow was markedly reduced not only in the hemorrhagic areas but also in the remainder of the involved hemisphere, suggesting that neurologic deficits may be caused by ischemia rather than the presence of blood within the brain parenchyma or cerebral ventricles (44). In full-term infants with perinatal asphyxia, diminished blood flow to the parasagittal cortical regions suggested that injury to the brain in these infants was also ischemic in etiology (45).
PET has also been employed to study the pathophysiology of many other childhood brain disorders such as autism (46), attention deficit hyperactivity disorder (47,48), mental retardation and developmental disabilities (49), age-associated metabolic changes in deafness (50), schizophrenia (51), sickle cell encephalopathy (52), and anorexia and bulimia nervosa (53,54), Rasmussen syndrome (55), Krabbe disease (56), Sturge-Weber syndrome (57), and cognitive impairment in Duchenne muscular dystrophy (58). However, the exact role of PET in these clinical settings remains unclear. Further experience may result in an expanded role of PET in many childhood neurological disorders.
PET in Pediatric Cardiology
Currently, PET plays a relatively minor role in pediatric cardiology. Quinlivan et al. (59) have reviewed the cardiac applications of PET in children. PET with nitrogen-13 ([13N])-ammonia has been employed to measure myocardial perfusion in infants after anatomic repair of congenital heart defects and after Norwood palliation for hypoplastic left heart syndrome (60). Infants with repaired heart disease had higher resting blood flow and less coronary flow reserve than previously reported for adults. Infants with Norwood palliation also had less perfusion and oxygen delivery to the systemic ventricle than the infants with repaired congenital heart lesion, explaining in part the less favorable outcome for patients with Norwood palliation. Evaluation of myocardial perfusion with [13N]-ammonia PET in infants following a neonatal arterial switch operation has demonstrated that patients with myocardial perfusion defects may have a more complicated postoperative course (61). The higher resolution of PET versus SPECT may have advantages in pediatric cardiac imaging, but this requires further study in specialized centers.
A major application of PET in adult cardiology is the assessment of myocardial viability with FDG as a tracer for glucose metabolism. Rickers et al. (62) evaluated regional glucose metabolism and contractile function by gated FDG PET in seven infants and seven children after arterial switch operation and suspected myocardial infarction. Gated FDG PET was found to contribute pertinent information to guide additional therapy including high-risk revascularization procedures. Other reports have also provided evidence for the utility of PET in the assessment of myocardial perfusion and viability in infants and children with coronary abnormalities (63,64).
In another study of children with Kawasaki disease, PET with [13N]-ammonia and FDG showed abnormalities in about 60% of patients during the acute and subacute stages and about 40% of patients in the convalescent stage of disease (65). PET was valuable in assessing the response to immunoglobulin therapy at differing doses and administration schedules. PET and ammonia may also reveal
reduction of coronary flow reserve in children with Kawasaki disease and angiographically normal epicardial coronary arteries (66).
reduction of coronary flow reserve in children with Kawasaki disease and angiographically normal epicardial coronary arteries (66).
Beyond the more common assessment of myocardial perfusion and oxidative metabolism, PET has been used to study such fundamental functional abnormalities as mitochondrial dysfunction in children with hypertrophic or dilated cardiomyopathy (67). Dynamic PET with [11C]-acetate demonstrated reduced myocardial Krebs cycle activity (i.e., decreased oxidative metabolism) in children with cardiomyopathy despite normal myocardial perfusion. The diminished oxidative metabolism was associated with compensatory increased glycolytic activity as demonstrated on FDG PET.
PET in Pediatric Oncology
The incidence of cancer is estimated to be 133.3 per million children in the United States (68). Although cancer is much less common in children than in adults, it is still an important cause of mortality in pediatrics. Approximately 10% of deaths during childhood are attributable to cancer, making it the leading cause of childhood death from disease (69).
Childhood cancers often differ from those encountered in adults. This is illustrated in Table 8.23.5, which delineates the estimated incidences of the more commonly encountered cancers in U.S. children. Of the adult cancers to which FDG PET has been most widely applied, only lymphomas and brain tumors occur with an appreciable incidence in children. However, the diagnostic utility of FDG PET and its impact on patient management have been reported for many pediatric cancers (70,71,72,73,74,75,76,77,78,79,80,81). In decreasing order of frequency, PET led to important changes in clinical management of lymphoma (32%), brain tumors (15%), and sarcomas (13%) (71).
Table 8.23.5 Cancer Incidence Rates Per Million Children Younger Than 15 Years in The United States as Derived from The Surveillance, Epidemiology, and End Results (SEER) Program | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PET/CT has also been shown to improve on PET alone by precise CT localization of metabolic abnormalities on PET and by metabolic characterization of abnormal and normal findings on CT, thereby increasing diagnostic confidence and reducing equivocal image interpretations (82,83,84,85). Before reviewing the applications of PET in pediatric oncology, it is important to consider potential causes of confusion on FDG PET that relate to physiologic FDG distribution in children. High FDG uptake by thymus, adenoids, tonsils, and skeletal growth centers (particularly the long bone physes) as well as the greater extent of hematopoietic marrow in the immature skeleton compared to the adult skeleton are important physiological variations in FDG distribution encountered in children (Figs. 8.23.3 and 8.23.4) (86,87,88,89,90). Specifically, the thymic FDG uptake appears to have a significant relationship with lymphocyte count and may be physiologically elevated after chemotherapy (Fig. 8.23.5) (91). Normal thymic uptake must be recognized to avoid confusion with mediastinal malignancy.
With the introduction of PET/CT imaging systems, it has been recognized that elevated FDG uptake in the normal brown adipose
tissue may also be a source of false-positive finding (92,93,94). The common anatomic areas involved include the neck and shoulder region, axillae, mediastinum, and the paravertebral and perinephric regions. Neck brown fat hypermetabolism is seen significantly more in the pediatric population than in the adult population (15% vs. 2% respectively; P <.01) and appears to be stimulated by cold temperatures (92,93). Recent data have shown that brown fat metabolic activity may be suppressed pharmacologically (e.g., propranolol, fentanyl) (95,96) or even more simply by controlling environmental temperature in the hours before injection and during the uptake phase (97).
tissue may also be a source of false-positive finding (92,93,94). The common anatomic areas involved include the neck and shoulder region, axillae, mediastinum, and the paravertebral and perinephric regions. Neck brown fat hypermetabolism is seen significantly more in the pediatric population than in the adult population (15% vs. 2% respectively; P <.01) and appears to be stimulated by cold temperatures (92,93). Recent data have shown that brown fat metabolic activity may be suppressed pharmacologically (e.g., propranolol, fentanyl) (95,96) or even more simply by controlling environmental temperature in the hours before injection and during the uptake phase (97).
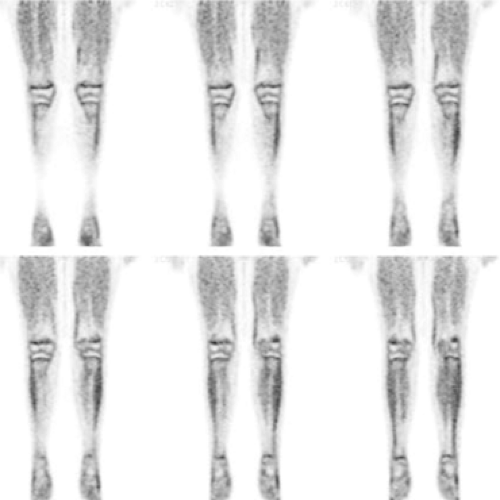 Figure 8.23.3. Coronal fluorodeoxyglucose PET images of the lower extremities of an 11-year-old boy with a history of Hodgkin’s lymphoma, off therapy for 3 years, show high physiologic uptake in the growth plates bilaterally.
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|