CHAPTER 8 Pelvic and lower extremity arteries
Arteriography
Bilateral lower extremity arteriography (“run-off” study) is done with the pigtail just above the aortic bifurcation. Serial images are obtained down to the feet. If only one leg needs to be examined, it is usual practice to catheterize the contralateral groin and direct a cobra or similarly shaped catheter over the aortic bifurcation (see Chapter 3). If a pigtail catheter is already in place, it can be gently unwound on the bifurcation and replaced with a straight catheter (see Fig. 3-16). A long, reverse-curve catheter simplifies entry into internal iliac artery branches (see Fig. 3-15).
When the tibial or pedal arteries are poorly visualized on the initial angiogram, they often are better seen by advancing a catheter into the common or superficial femoral artery and first injecting an intraarterial vasodilator (e.g., 100 to 200 μg of nitroglycerin). In patients with a history of severe contrast allergy or renal insufficiency, alternative noninvasive imaging should be attempted. If catheter angiography is required before treatment, carbon dioxide can be used exclusively or supplemented with small volumes of iodinated contrast1–4 (see Chapter 3).
Anatomy
Development
In the embryo, the lower extremities are supplied by the axial artery, which arises from the sciatic branch of the internal iliac artery.5 This vessel ends in a plantar network in the developing foot. The femoral artery, which runs along the ventral aspect of the limb, is the continuation of the external iliac artery; it joins the axial artery at the knee to form the popliteal artery. The posterior tibial and peroneal arteries originate from the axial artery below the knee and run along the dorsal aspect of the calf. The anterior tibial artery takes off from the lower popliteal artery and courses along the ventral aspect of the calf. The superficial femoral artery eventually becomes the dominant vessel to the lower leg. The deep femoral artery arises near the bottom of the femoral head. Most of the axial artery regresses before birth; normally, the only remnants are portions of the inferior gluteal, popliteal, and peroneal arteries.
Normal anatomy
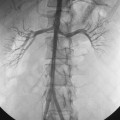
The abdominal aorta divides into the common iliac arteries at the L4-L5 level6 (Figs. 8-1A and 8-2). The common iliac arteries lie in front of the iliac veins and the inferior vena cava. They usually have no major branches; rarely, they give off aberrant iliolumbar or accessory renal arteries. The common iliac artery divides near the lumbosacral junction. The external iliac artery continues directly to the groin behind the inguinal ligament. This vessel also has no major branches. The internal iliac artery takes off medially and posteriorly. At the superior edge of the greater sciatic foramen, it usually divides into anterior and posterior trunks. The branching pattern of the internal iliac artery is quite variable (see Fig. 8-1B). Classically, the major branches of the anterior division are as follows:
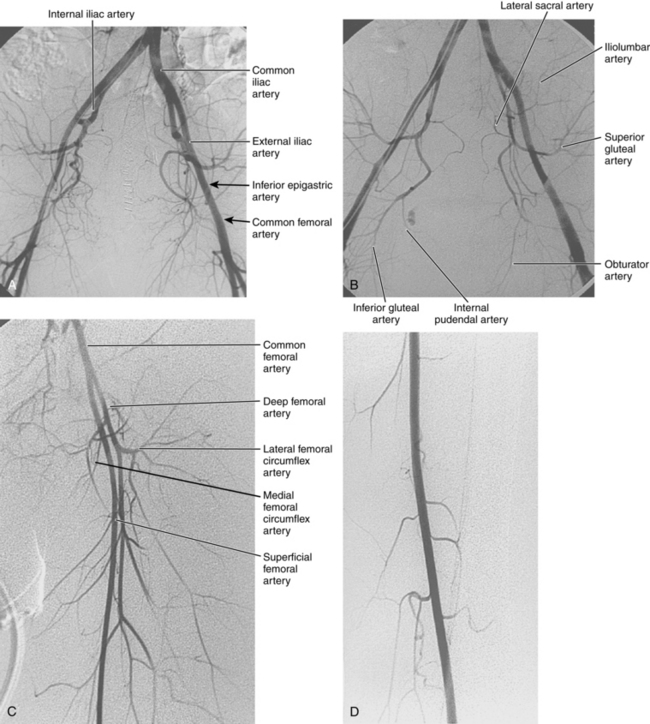
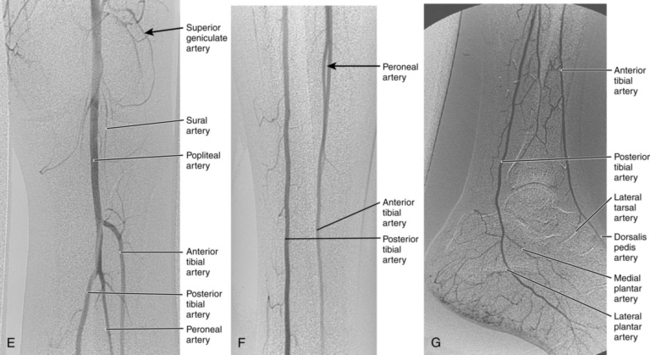
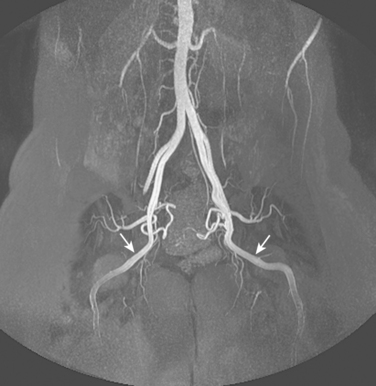
Figure 8-1 Normal pelvic and left lower extremity arteriograms. A, The abdominal aorta divides into the common iliac arteries at the L4-L5 level. B, The branching pattern of the internal iliac artery varies. C, The common femoral artery divides into the superficial femoral artery (SFA) and deep femoral artery near the bottom of the femoral head. D, The SFA passes down the anteromedial aspect of the thigh, dives into the flexor muscle compartment, and runs through the adductor (Hunter) canal. E and F, At the distal border of the popliteus muscle, the popliteal artery divides. G, The dorsalis pedis artery gives off medial and lateral tarsal branches. The posterior tibial artery gives off medial and lateral plantar branches.
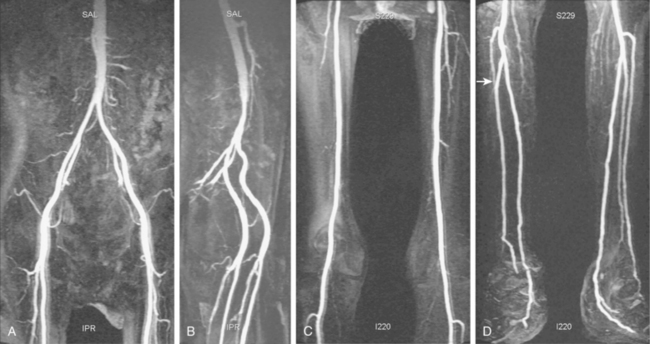
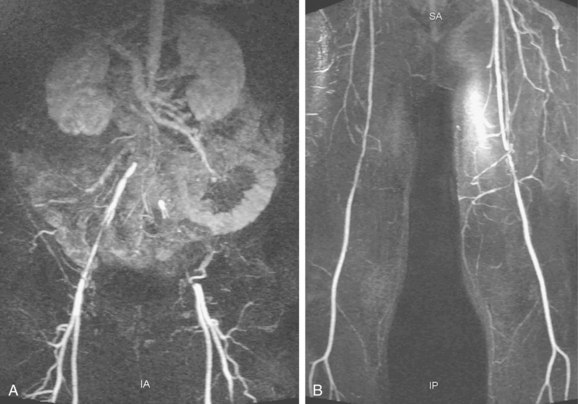
Figure 8-2 Pelvic and lower extremity maximum intensity projection magnetic resonance angiogram. Pelvis in frontal (A) and near lateral (B) views. Thigh (C) and calf (D) in frontal view. The right anterior tibial artery is occluded in the upper calf (arrow).
The major branches of the posterior division of the internal iliac artery are:
At the junction of the external iliac and common femoral arteries (which corresponds to the inguinal ligament), the inferior epigastric artery exits medially (see Fig. 3-1). It runs alongside the rectus abdominis muscle before communicating with the superior epigastric branch of the internal thoracic (mammary) artery. The deep iliac circumflex artery takes off laterally and superiorly (see Fig. 3-5).
The common femoral artery (CFA) courses over the femoral head encased in the femoral sheath along with the femoral vein (medial or posteromedial) and the femoral nerve (lateral). Branches of the CFA include the superficial epigastric artery, superficial circumflex iliac artery (laterally), and external pudendal artery (medially); all of these are inconsistently seen at angiography. The CFA divides into the superficial femoral artery (SFA) and deep femoral artery (DFA) or profunda femoris artery (PFA) near the bottom of the femoral head (see Fig. 8-1C). A “high” bifurcation is occasionally seen. The DFA takes off laterally and posteriorly. Its major branches are the lateral femoral circumflex, medial femoral circumflex, and four or so pairs of perforating arteries.
The SFA passes down the anteromedial aspect of the thigh, dives into the flexor muscle compartment, and runs through the adductor (Hunter) canal (see Fig. 8-1D). The SFA then becomes the popliteal artery, which is posterior to the femur (surrounded by the heads of the gastrocnemius muscle) and deep to the popliteal vein. Its major muscular branches are the sural arteries and paired superior, middle, and inferior geniculate arteries, all of which form an anastomotic network around the knee.
At the distal border of the popliteus muscle, the popliteal artery divides.7 The anterior tibial artery arises laterally, pierces the interosseous membrane, and then runs in front of the lower tibia (see Fig. 8-1E and F). It passes over the ankle onto the dorsum of the foot to become the dorsalis pedis artery. The tibioperoneal trunk is the direct continuation of the popliteal artery and bifurcates just beyond its origin into the posterior tibial and peroneal arteries. The posterior tibial artery runs posteriorly and medially in the flexor compartment. The peroneal artery runs between the posterior and anterior tibial arteries near the fibula. It is a small-caliber vessel unless functioning as a collateral in the face of tibial artery obstruction. In the distal calf, its perforating and communicating branches may join the anterior and posterior tibial arteries, respectively. Above the ankle, the artery divides into two calcaneal branches (“fish tail”) that have anastomoses with the distal tibial arteries.
A network of malleolar arteries interconnect the tibial arteries above the ankle.7 The dorsalis pedis artery gives off medial and lateral tarsal branches (see Fig. 8-1G). The posterior tibial artery passes behind the medial malleolus, where it divides into medial and lateral plantar arteries. The plantar arch is formed by the dominant lateral plantar branch of the posterior tibial artery and the distal dorsalis pedis artery. Smaller secondary arches are created by other branches of the distal tibial arteries. Metatarsal arteries arise primarily from the plantar arch.
Variant anatomy (online case 102)
The persistent sciatic artery is a rare anomaly (about 0.1% of the population) in which the embryologic sciatic artery remains the dominant inflow vessel to the leg.8,9 The aberrant vessel arises from the internal iliac artery, passes through the greater sciatic foramen, and lies deep to the gluteus maximus muscle (Fig. 8-3). Above the knee, it joins the popliteal artery. The SFA is hypoplastic or absent. The anomaly is occasionally bilateral. Because of its relatively superficial position in the ischial region, the sciatic artery is prone to intimal injury or aneurysm formation.
Very rare femoral artery variants include the saphenous artery and duplication of the SFA.10 Anomalies of the DFA are common, including a posterior or even medial origin of the main DFA trunk and separate origins of the medial and lateral circumflex femoral branches.
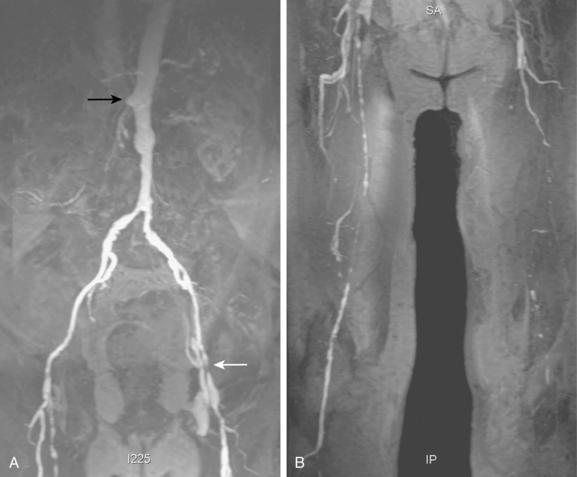
Tibial artery anomalies are present in about 3% to 10% of the population.11–13 The variants often are bilateral. The most frequent are “high” bifurcation or true trifurcation of the popliteal artery, common origin of the anterior tibial and peroneal arteries, and hypoplasia or absence of the anterior or posterior tibial artery (Fig. 8-4). In the latter case, the affected vessel is normal at its origin but gradually tapers in the mid to distal calf without discrete termination. A major branch of the peroneal artery often reforms the absent vessel above the ankle, resulting in normal pedal pulses.
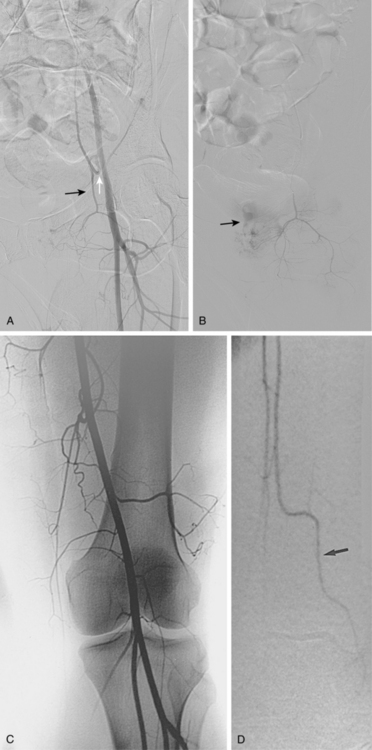
Figure 8-4 A, Obturator artery (black arrow) arises from the root of the inferior epigastric branch of the external iliac artery (white arrow) instead of the internal iliac artery. B, Extravasation of contrast (arrow) is due to pelvic trauma. Lower extremity arterial variants C, High bifurcation of the popliteal artery with common origin of the anterior tibial and peroneal arteries. D, Absence of the right posterior tibial artery, which is reformed distally by a congenitally enlarged branch of the peroneal artery (arrow).
Collateral circulation
The pelvis and lower extremities have rich and complex systems of collateral circulation that maintain blood flow to the leg when proximal arteries are obstructed. The major routes are formed by branches of the internal iliac, deep femoral, and popliteal arteries (Fig. 8-5 and see Fig. 7-5):
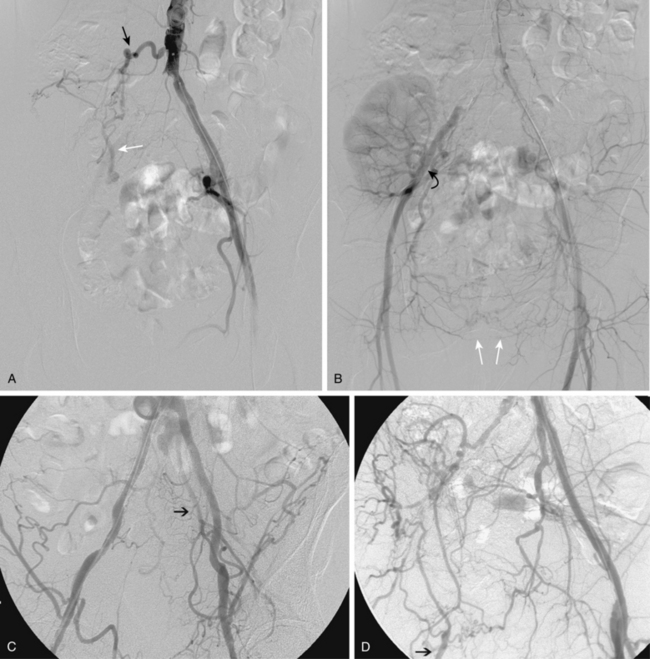
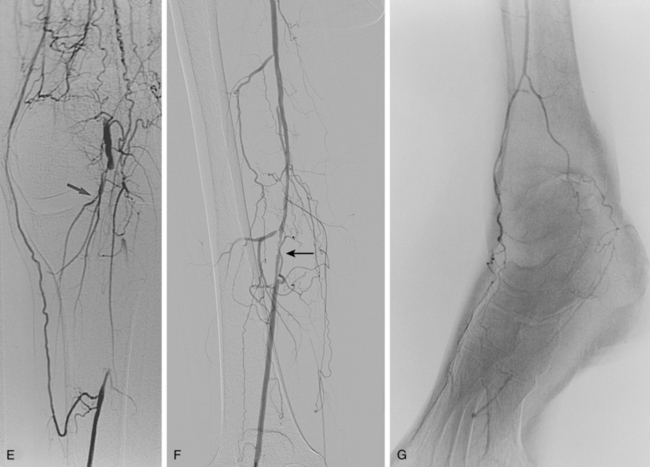
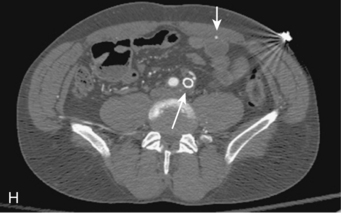
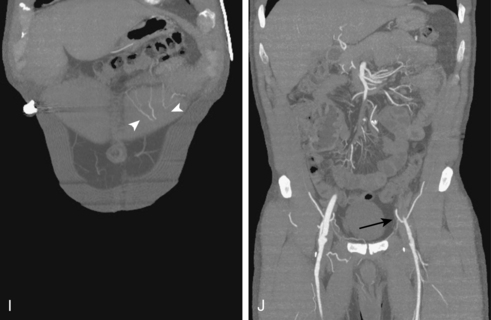
Figure 8-5 Collateral patterns in cases of lower extremity arterial obstructions (see text for details). A and B, Right common iliac artery (CIA) occlusion with major collateral circulation through the fourth lumbar artery (black arrow) to the iliolumbar branch of the internal iliac artery (IIA, white arrow) and then into the external iliac artery (EIA, curved arrow). There are also transpelvic collaterals from left to right IIA (double white arrows). Note renal transplant off the right EIA. C, Bilateral IIA occlusions. In addition to distal external iliac/common femoral collaterals, the enlarged inferior mesenteric artery (arrow) contributes to the IIA branches. D, Right external iliac artery occlusion with reconstitution of the proximal deep femoral artery (arrow). E, Left femoropopliteal and proximal tibial artery occlusions. Deep femoral branches reconstitute a short segment of the midpopliteal artery, which supplies sural collaterals (arrow) into the calf. The posterior tibial artery is reconstituted in the midcalf. F, Critical stenosis of distal right superficial femoral artery (arrow) with multiple collateral channels. G, Posterior and anterior tibial artery occlusion, with the peroneal artery reconstituting the distal vessels in the foot. H through J, Thrombosis of left limb (long white arrow) of aortobifemoral bypass graft on axial and reformatted coronal contrast enhanced computed tomography images. The left leg is partially fed through superficial epigastric branches (arrowheads) of the distal left internal thoracic (mammary) artery. These vessels communicate with branches of the inferior epigastric artery (black arrow) which then fill the left common femoral artery.
Major disorders
Chronic peripheral arterial disease (online case 2)
Etiology
Obstructive disease of the pelvic and lower extremity arteries is one of the most frequent clinical problems encountered by interventionalists. In the United States, the overall prevalence is 3% to 10% of the general population; up to 20% of individuals older than age 70 suffer from this disorder.14,15 The cardinal feature is diminished blood flow to the pelvis or legs with exercise or at rest.
Peripheral arterial disease (PAD) encompasses the following three clinical scenarios:
There are a variety of causes for lower extremity PAD (Box 8-1). However, the vast majority of cases result from atherosclerotic stenoses, superimposed thrombosis, or embolism. By a wide margin, atherosclerosis is the most common disease affecting the lower extremity arteries. Most of the established risk factors are well known14,16 (Box 8-2). The pathophysiology of atherosclerosis and the emerging significance of certain inflammatory markers (e.g., C-reactive protein) in progression of disease and response to treatment are discussed in Chapter 1. In certain situations, the interventionalist should keep in mind the less common causes of PAD (Box 8-3).
Atherosclerosis is a systemic disease. As such, it is typically diffuse and bilateral. However, there is a clear predilection for clinically significant disease in the distal aorta, common and external iliac arteries, distal SFA, and tibial arteries. The propensity for obstructions in the distal SFA as it passes through the adductor canal is related to turbulent flow and altered wall shear stress. These hemodynamic disturbances result from changes in curvature and tortuosity of this mobile vessel along with compression on the vessel by the adductor magnus muscle and fascia at the adductor canal.17,18 Atherosclerotic plaques cause symptoms by impeding blood flow to the leg, inducing thrombotic occlusion, or through embolization of clot or plaque fragments. In CLI, obstructions are accompanied by abnormalities of skin microcirculation that further exacerbate ischemia.
Macroemboli usually are thrombi that originate from the heart. Less often, clot or plaque fragments break off from aneurysms or atherosclerotic surfaces in the proximal arteries. Emboli usually lodge at branch points or sites of underlying disease. Microemboli are composed of platelet-fibrin deposits or cholesterol crystals arising from atherosclerotic plaques or aneurysms. These particles may be released spontaneously or during operative manipulation or catheterization. Microemboli can lead to blue toe syndrome or an acute cholesterol embolization event (see Chapter 1).
Clinical features
By definition, the diagnosis of chronic PAD requires symptoms lasting more than 2 weeks. The severity of PAD often is graded according to the scale of Rutherford and Becker19 (Table 8-1). At least 50% of patients with PAD are asymptomatic or have atypical symptoms; in some cases, limited physical activity prevents the onset of leg pain. Only 10% or so will ultimately develop CLI.14 Chronic PAD is unusual before age 40. Men are affected more frequently than women, as are nonwhite individuals.14 Most patients have one or more risk factors for disease (see Box 8-2). These patients usually suffer from associated coronary artery disease, cerebrovascular disease, or chronic renal insufficiency. PAD is an important marker of these conditions.
Table 8-1 Rutherford-Becker Classification of Peripheral Arterial Disease
| Grade | Category | Symptoms |
|---|---|---|
| 0 | 0 | None |
| I | 1 | Mild claudication |
| I | 2 | Moderate claudication |
| I | 3 | Severe (lifestyle-limiting) claudication |
| II | 4 | Rest pain |
| III | 5 | Nonhealing ulcers, focal gangrene |
| III | 6 | Major tissue loss |
Intermittent claudication (IC) is the first symptom in some patients. IC is characterized by predictable and reproducible calf, thigh, or buttock muscle pain or fatigue with exercise (especially walking on an incline) that is invariably relieved by rest. The pain does not resolve with continued leg exercise. It rarely occurs in the foot. Unfortunately, a variety of unrelated conditions are often confused with true claudication, including spinal stenosis, nerve root compression, arthritis, chronic compartment syndrome, and venous claudication.14,20 Still, a careful history will usually distinguish among these entities. In a minority of afflicted patients, the collateral circulation eventually becomes insufficient to prevent muscle ischemia at rest despite maximum peripheral vasodilation. Chronic limb ischemia is present when symptoms of rest pain or ischemic skin changes are combined with an ankle-brachial index less than 0.50 (see later discussion).
Ischemic skin changes may follow if the obstructions worsen or revascularization is not done. Ischemic ulcers often start with trivial skin injury, which is particularly dangerous in patients with diabetes with peripheral neuropathy and altered sensation. The toes are most affected, although arterial heel and malleolar ulcers do occur. Again, ischemic ulcers must be distinguished from traumatic, venous, or neuropathic lesions.14 Their appearance is sometimes distinctive: located on the toes (or heel), dry with a pale base, sharply marginated, and associated with severe pain. Ulcers about the malleoli or above the ankle are usually venous in origin (see Chapter 15). Gangrene is the feared sequela of CLI. Amputation may then be necessary. Diabetic patients suffer a major amputation rate that is 5- to 10-fold greater than the general PAD population.14
Patients with blue toe syndrome complain of relatively acute onset of painful, bluish-colored toes on one or both feet.21,22 The symptoms often resolve but may recur and occasionally lead to tissue loss. The peripheral pulses often are intact.
Natural history
However, once CLI is present, the overall prognosis is dismal. Within 1 year, 25% of such patients will require major amputation and another 25% will be dead. These disturbing figures reflect the nature of PAD: a systemic vascular disease with a very high mortality rate, largely from associated coronary artery disease or stroke (i.e., 30% at 5 years, 50% at 10 years).14,15 Almost every patient who suffers from CLI is dead within a decade.
Noninvasive testing
The ankle-brachial index (ABI) is extremely sensitive (95%) and specific (almost 100%) for the diagnosis of PAD.14,23 It is an absolutely essential part of the evaluation of all patients with known or suspected PAD. The systolic blood pressure at the ankle is divided by the systolic brachial arterial pressure to yield an index (Table 8-2). Rest pain usually requires an ABI less than 0.5 or an absolute ankle pressure less than 50 mm Hg. Ischemic ulcers can develop below a pressure of 50 to 70 mm Hg. However, calcified or noncompressible arteries (as found in patients with diabetes or chronic renal failure) falsely elevate pressure measurements and can lead to underestimation of disease severity. In this situation, toe pressure measurements are more useful. The toe index is normally greater than 0.60. An absolute toe pressure of greater than 30 mm Hg is generally required for wound healing.
Table 8-2 Ankle-Brachial Index Classifications
| Range | Rutherford Grade | Disease |
|---|---|---|
| <0.5 | II or III | Chronic limb ischemia |
| 0.51 to 0.90 | I | Intermittent claudication |
| 0.91 to 1.3 | 0 | No significant PAD |
| >1.4 | — | Noncompressible vessels, likely to have PAD |
PAD, peripheral artery disease.
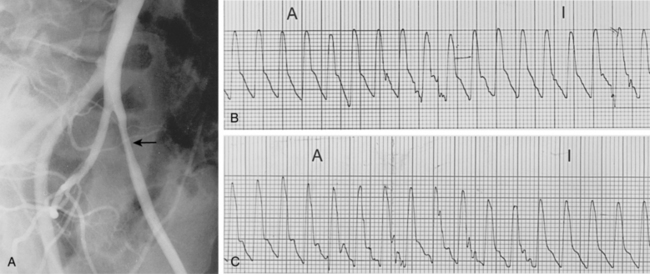
Segmental blood pressures are obtained by sequentially inflating blood pressure cuffs around the upper thigh, lower thigh, calf, ankle, and toe. Several sets of guidelines have been established to interpret these values.14,23 A drop of 20 to 30 mm Hg between levels (or comparing legs at the same level) suggests a significant stenosis or occlusion (Fig. 8-6A). Abnormal values at the upper thigh, lower thigh, calf, and ankle reflect obstructions in the aortoiliac segments, SFA, femoropopliteal segment, and tibial arteries, respectively (see Fig. 8-6B). However, the correlation is not always precise; for example, proximal femoral artery disease can mimic aortoiliac disease. Segmental pressure measurements after exercise can detect occult disease in patients with suspicious symptoms and a normal study at rest (see Fig. 8-6C). While walking on an inclined treadmill, ankle and brachial pressure measurements are periodically recorded. A fall in ankle pressure is diagnostic of significant PAD.
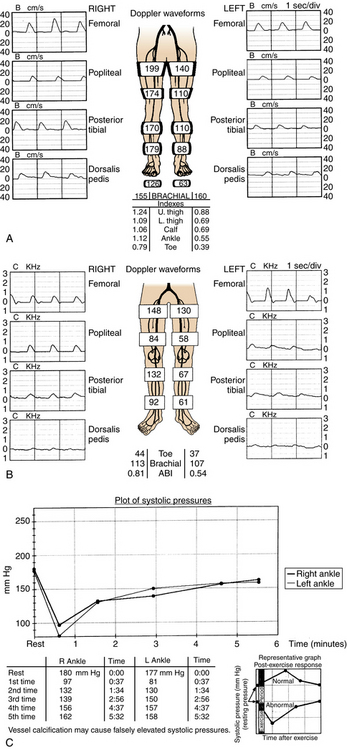
Figure 8-6 Segmental blood pressure measurements. A, Abnormal left upper thigh index (0.88) in a patient with left leg claudication. The angiogram showed a tight left common iliac artery stenosis. B, Bilateral drop in lower thigh pressures in a patient with bilateral leg claudication. Notice dampening of the normal waveform at this level. Angiography showed bilateral occlusions of the superficial femoral arteries. C, After exercise, the ankle pressures dropped abnormally in a patient with bilateral calf claudication and normal resting segmental pressures. The angiogram demonstrated bilateral tibial artery disease.
Plethysmography (pulse volume recordings, PVR) utilizes changes in leg volume to reflect overall perfusion of the limb.23 With mild or moderate PAD, digital or segmental pulse volume tracings are dampened; with severe occlusive disease, the waveform is almost flat. This technique is particularly useful when segmental pressure measurements are inaccurate, as in patients with noncompliant arteries.
Imaging
Color doppler sonography
Color Doppler (“duplex”) sonography is favored in some laboratories for arterial mapping to depict and grade arterial stenoses and occlusions. The technique is sensitive (∼80% to 90%) and quite specific (∼95%).23,24 The normal Doppler spectral pattern in these high-resistance arteries is triphasic, with rapid forward flow in systole followed by brief reversal of flow in early diastole. Direct and indirect indicators of significant PAD are listed in Box 8-4.
Magnetic resonance angiography
Magnetic resonance (MR) angiography has revolutionized the evaluation of patients with PAD.25–28 When properly performed, MR angiography provides images almost comparable in quality and form to conventional catheter angiography with none of the associated risks (see Fig. 8-2). The sensitivity and specificity of state-of-the-art MR angiography approaches or exceeds 95%26–30 (Figs. 8-7 and 8-8).
MR angiography is contraindicated in certain patients (e.g., those with pacemakers, intracranial clips, severe claustrophobia). Each institution must develop its own protocol for routine imaging of patients with PAD, and software and hardware are constantly evolving. The gadolinium injections and imaging acquisitions are coordinated such that the imaging interval is centered around the time of peak gadolinium concentration. Initial unenhanced two-dimensional time-of-flight acquisitions can be helpful in evaluating the calf and pedal vessels, but add substantial time to the examination. In addition to inspecting the maximum intensity projections in multiple planes, it is crucial to review source data to confirm findings.
Computed tomography angiography
The widespread availability of multidetector computed tomography (CT) scanners has made CT angiography an important tool in evaluation of a wide variety of vascular diseases, including PAD31 (Fig. 8-9). The sensitivity and specificity with current technology is about 92% to 96% compared with catheter digital angiography.32–35 The major pitfalls of CT angiography for PAD include pulsation artifacts (which are minimized with cardiac gating), contrast bolus mistiming, calcified vessels, and the presence of metallic objects (e.g., stents, coils) (Fig. 8-10).
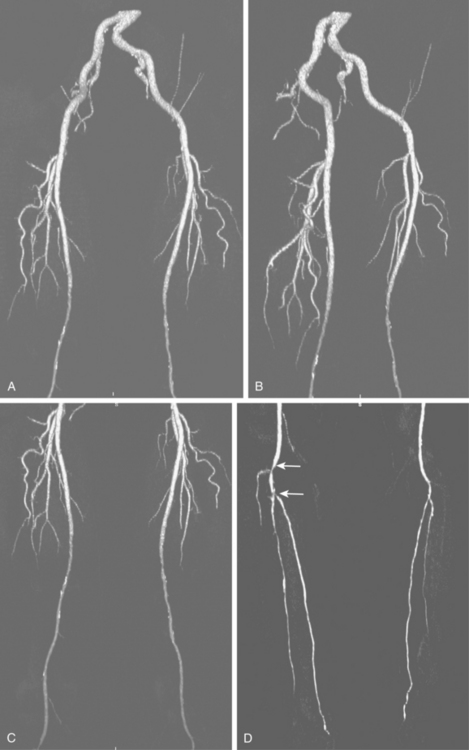
Figure 8-9 Pelvic and extremity arteries at computed tomography angiography, including the pelvis (A and B), thigh (C), and calf (D). Diffuse bilateral mid superficial femoral artery (SFA) disease is present. There are right distal popliteal and tibioperoneal trunk stenoses (white arrows). Neither anterior tibial artery is opacified.
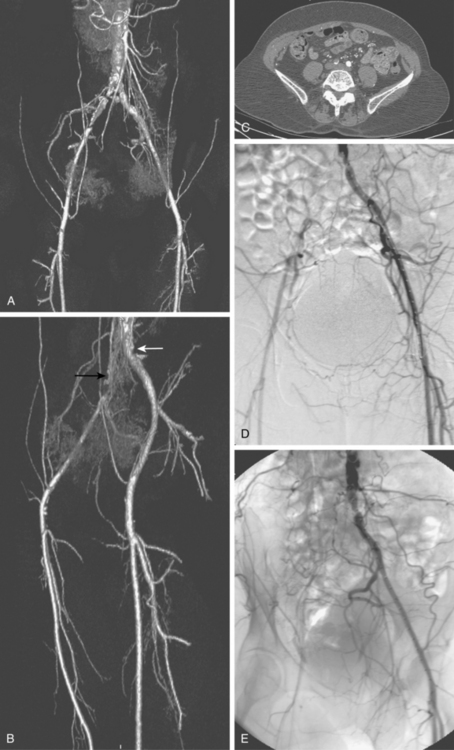
Figure 8-10 Correlation of computed tomography and catheter angiography for peripheral vascular disease. Frontal (A) and oblique (B) shaded-surface display computed tomography reconstructions suggest complete right common iliac artery occlusion (black arrow), and moderate left common iliac artery stenosis (white arrow). The former is confirmed on review of axial images (C). Note that significant calcification partially obscures the occlusion on reformatted images. The findings were confirmed on a catheter angiogram in frontal (D) and left posterior oblique (E) projections.
Catheter angiography
Visualization of the entire arterial circulation to the foot is mandatory because vascular surgical techniques allow bypass to the pedal arteries. Measurement of aortoiliac pressure gradients after vasodilator injection is particularly important in patients with claudication or before infrainguinal bypass graft placement (Fig. 8-12). The drop in peripheral vascular resistance and resulting increase in flow that occurs with exercise or after graft placement increase the significance of proximal stenoses (see Chapter 3).
Certain points in the interpretation of lower extremity run-off studies are worth comment:
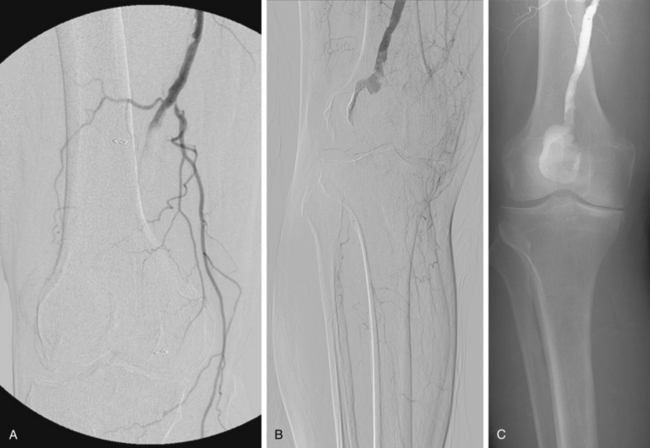
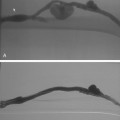
Figure 8-14 Right popliteal artery aneurysm. A, Initial right leg arteriogram shows complete occlusion of the upper popliteal artery. An aneurysm was not initially suspected. B and C, After partial thrombolysis, the aneurysm becomes apparent.
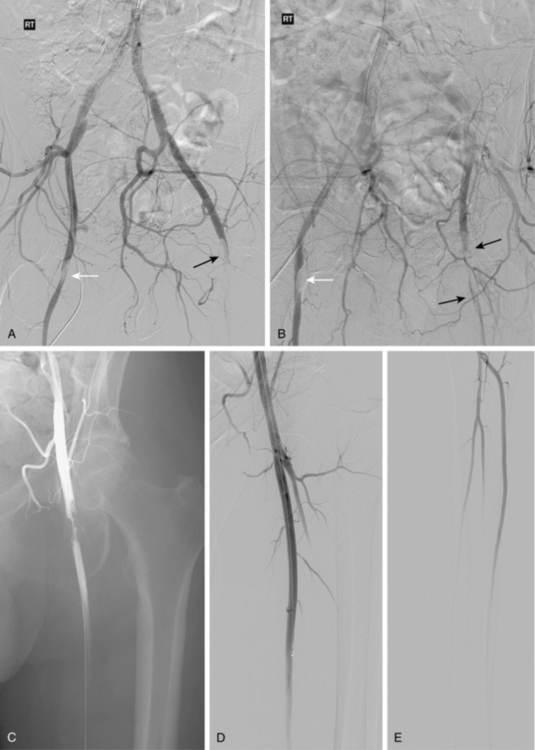
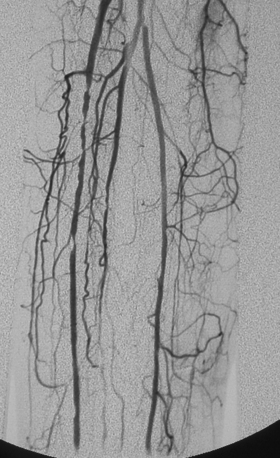
Figure 8-16 Embolism to both common femoral arteries (CFA) with severe left leg ischemia. A and B, Bilateral oblique pelvic arteriography shows a near occlusive embolus to the right CFA (white arrow). The right deep femoral artery is blocked. A left CFA embolus is almost completely obstructive and extends into the superficial and deep femoral arteries (black arrow). Blood flows from internal iliac artery collaterals into deep femoral artery branches. C, Following initial treatment with 12 mg of t-PA, there is some lysis of clot. D and E, Following additional thrombolytic therapy, the embolus is completely resolved with three vessel run-off in the calf.
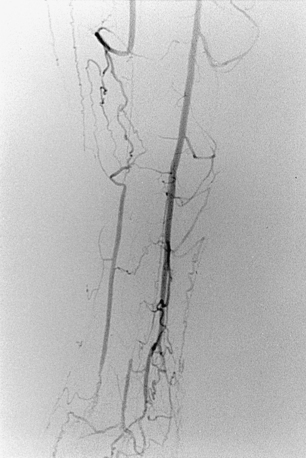
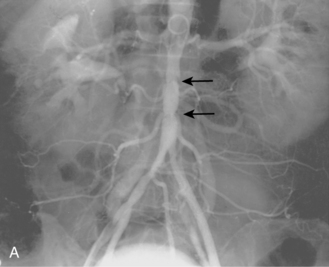
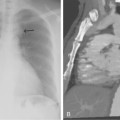
Figure 8-20 Radiation arteritis in a woman with a remote history of radiation therapy for cervical cancer and new right buttock claudication. A and B, Shaded-surface display reformatted CT angiography shows entirely normal lower extremity arteries except left internal iliac artery (IIA) occlusion and a moderate origin stenosis of the right internal iliac artery (arrow). Pelvic arteriogram (C) and selective right common iliac arteriogram in left anterior oblique projection (D) confirm these findings and show enlarged lumbar arteries providing collateral circulation into the pelvis. E, Angioplasty of the right IIA gave a good technical result but did not improve symptoms, probably due to radiation-induced small vessel arteritis.
Medical therapy
Many patients with asymptomatic PAD or intermittent claudication respond to medical therapy without the need for any type of imaging or intervention. The treatment goals are to stabilize or reduce lower extremity symptoms and prevent other cardiovascular events (e.g., myocardial infarction or stroke). The interventionalist must work aggressively with the patient to accomplish these goals14,36 (Box 8-5). Drug therapy, risk factor modification, and a supervised exercise program remain the cornerstones of treatment for mild to moderate IC. Endovascular interventions should serve an adjunctive role.37,38
Box 8-5 Conservative Management of Peripheral Arterial Disease
A host of pharmacologic agents have been tried for specific treatment of PAD. Most are not effective, including pentoxifylline, aspirin, vasodilators, and l-arginine.14 Two drugs are valuable in patients with IC. Cilostazol is a phosphodiesterase-III inhibitor with several properties of direct benefit to claudicants.39,40 There is strong evidence that long-term use will significantly increase walking distance and improve overall quality of life in this population. Side effects include diarrhea, headaches and palpitations. Cilostazol is contraindicated in patients with congestive heart failure. Naftidrofuryl is a 5-hydroxytryptamine antagonist the inhibits red cell and platelet aggregation and enhances striated muscle metabolism. Daily therapy affords similar benefits to cilostazol.41–43
For patients with CLI, the goals of therapy are relief of pain, healing of ulcers, avoidance of major amputation, and improvement in quality of life. Narcotic analgesics are appropriate before revascularization or for palliation. Along with aggressive antibiotic therapy for infections, local would care is essential.
Endovascular therapy
Patient selection
Selection of patients for endovascular versus open surgical therapy depends on a variety of patient factors and the site and nature of disease. For almost all arterial segments, results are better in groups suffering from claudication than those with CLI. The guidelines of the TransAtlantic Inter-Society Consensus on Peripheral Arterial Disease (TASC) working group are widely recognized14 (Fig. 8-22).
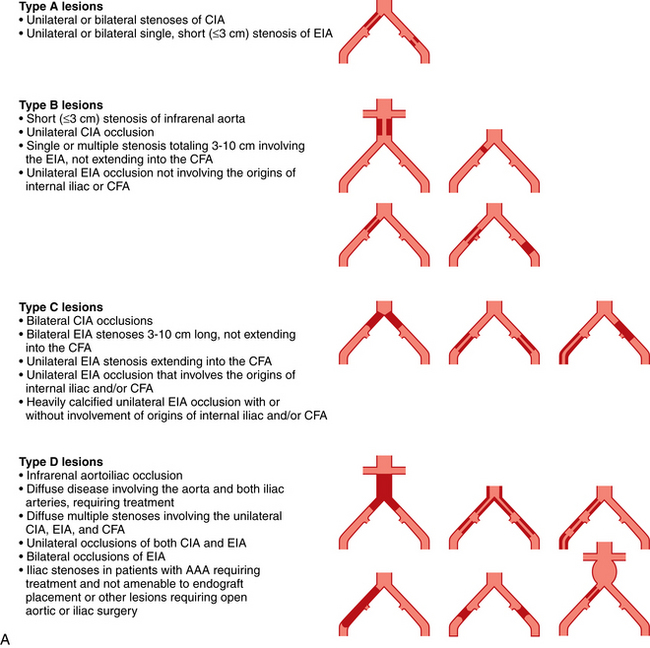
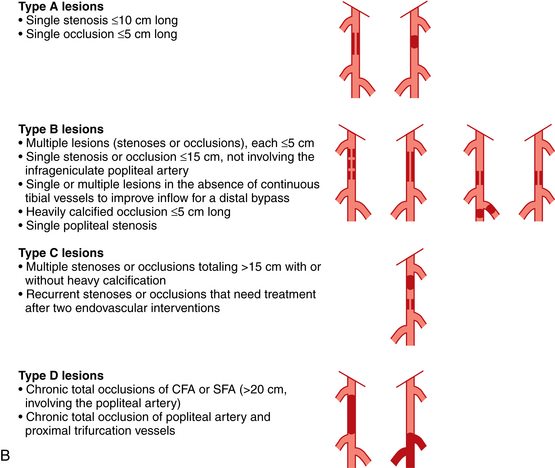
Figure 8-22 A, TASC II classification of aortoiliac lesions. B, TASC II classification of femoropopliteal lesions.
(Adapted from Norgren L, Hiatt WR, Dormandy JA, et al: Inter-society consensus for the management of peripheral arterial disease [TASC II]. Eur J Vasc Endovasc Surg 2007;33:S1.)
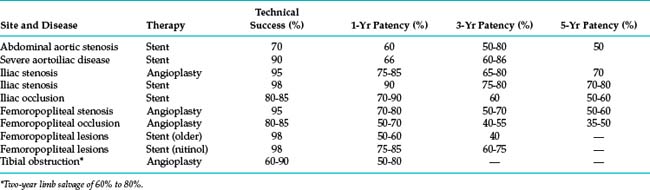
The technical details of percutaneous arterial revascularization are considered in detail in Chapter 3. Selection criteria, specific technical features, and outcomes at various sites are discussed herein. Perhaps in no other area of interventional radiology are reports of the efficacy of a procedure more disparate and confusing. Older radiologic series focused primarily on technical success and patency rates. The outcomes summarized in Table 8-3 are rough estimates intended only to help educate patients and referring physicians. To properly compare endovascular procedures with surgical treatment, these modalities must be evaluated in terms of durable clinical improvement in ischemic symptoms, limb salvage, and survival.
It is interesting to note that arterial angioplasty and stent placement provoke an inflammatory reaction at the treated site. The response is marked by elevations of serum C-reactive protein and fibrinogen (among other factors) and is more intense after femoropopliteal percutaneous transluminal balloon angioplasty (PTA) than carotid or iliac artery PTA. This phenomenon may partly explain the increased frequency of clinical restenosis at this site.45 The risk of restenosis also may be associated with higher preprocedure and postprocedure levels of C-reactive protein.46,47
Infrarenal aortic stenosis (TASC b lesions)
Isolated, severe atherosclerotic abdominal aortic stenoses are relatively uncommon. They occur mostly frequently in middle-aged or elderly women who smoke and in patients with hypoplastic aortic syndrome (small aorta and hypoplastic iliofemoral arteries).48 The traditional therapy was surgical endarterectomy or bypass grafting. For endovascular repair, these lesions cause some trepidation for interventionalists because the theoretical risk of rupture is greater than at other sites due to the large diameter of the aorta. In reality, angioplasty is remarkably safe, effective, and durable at this location49 (Fig. 8-23 and see Table 8-3). Primary hemodynamic patency at 10 years with PTA alone is close to 50%. Stents are certainly justified for failures of angioplasty and for large exophytic lesions that are more likely to shower emboli with balloon dilation.50 In actual practice, and despite the lack of solid evidence, most interventionalists opt for primary stent placement for all infrarenal aortic stenoses.51–55 In one small series, 3-year primary patency was 83% with primary stent insertion.53
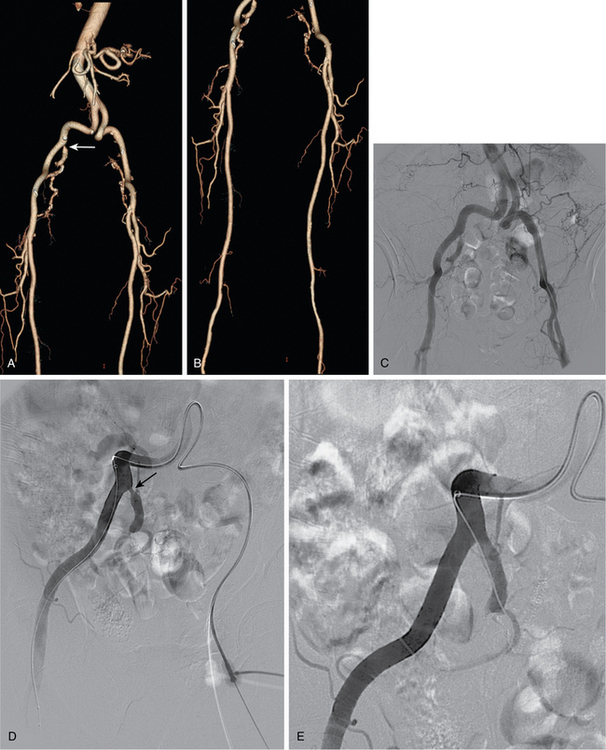
Aortic occlusion and severe aortoiliac occlusive disease (TASC c and d lesions)
This relatively uncommon form of PAD is typically seen in a somewhat younger population, most of whom smoke.56 Symptoms of bilateral claudication (and impotence in men) are common (Leriche syndrome) (see Fig. 7-5). The singular risk of an untreated infrarenal aortic occlusion is proximal propagation of clot leading to renal or mesenteric artery thrombosis. For these TASC C and D lesions, first-line treatment is still aortobifemoral bypass (AFB) grafting. Inferior alternatives include endarterectomy, axillobifemoral grafting, or thoracoaortic femoral grafting. Recently, some aggressive interventionalists have tackled these difficult cases by endovascular reconstruction with (limited) thrombolysis, angioplasty, and stent insertion (bare or covered). Long-term patency is still inferior to AFB grafting, but is perhaps acceptable given the reduced risk of perioperative complications, similar amputation rates, and comparable 30-day mortality.57–62 The reported primary patency rates at 3 and 5 years are 66% and 60% to 86%, respectively, with secondary patency rates of 90% and 80% to 98%, respectively. Hybrid interventions are becoming increasingly popular, whereby suprainguinal endovascular treatment is done in concert with CFA endarterectomy and/or infrainguinal bypass grafting 63
Iliac artery stenosis (TASC a-d lesions)
Primary stent placement is now standard of care for all TASC type B and C iliac lesions.57,64 Although formally considered operative territory, many interventionalists now handle type D iliac lesions. Balloon angioplasty is effective and durable for many simple iliac artery stenoses (Table 8-4). The oft-quoted Dutch Iliac Stent Trial and some other reports found no particular benefit to primary stent placement over selective stenting for type A and B lesions.65,66 Even so, many experienced operators still prefer primary stent placement for all iliac artery stenoses. Primary stent placement is certainly justified in the following situations67 (Fig. 8-24).
Table 8-4 Ideal Conditions for Balloon Angioplasty of Lower Extremity Arteries
| Lesion Characteristics | Patient Characteristics |
|---|---|
| Short | Nondiabetic |
| Concentric | Milder degrees of ischemia |
| Noncalcified | |
| Solitary | |
| Nonocclusive | |
| Large vessel | |
| Continuous in-line run-off |
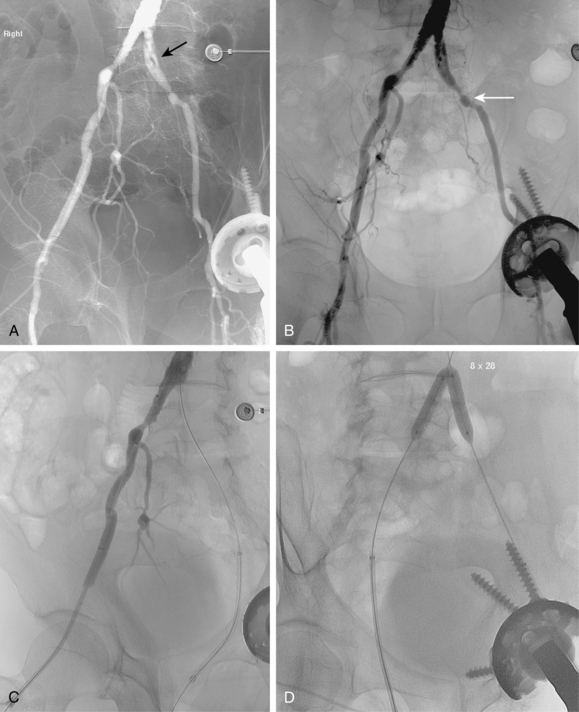
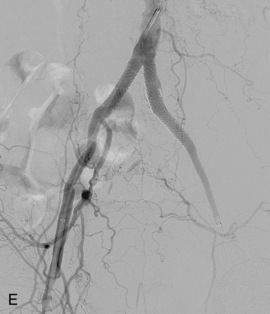
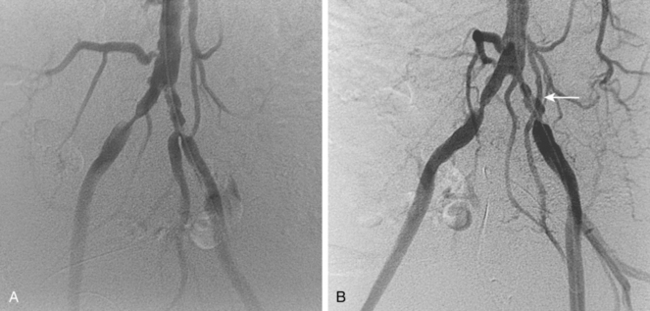
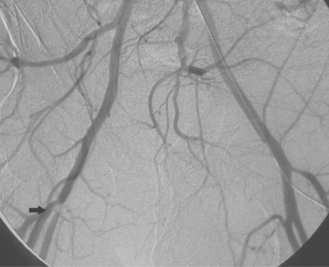
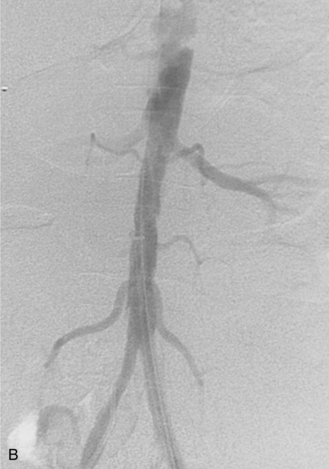
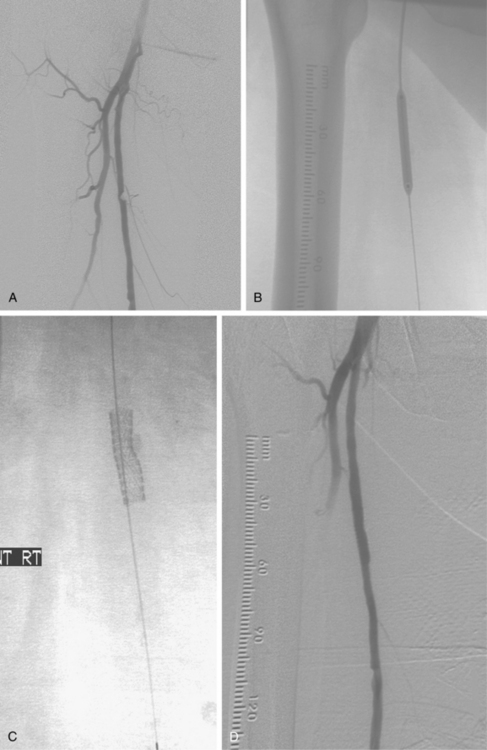
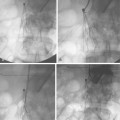
Figure 8-24 Bilateral iliac artery stent insertion. A and B, Right posterior oblique and frontal pelvic arteriography show a right common iliac artery stenosis, weblike left common iliac artery stenosis (black arrow), and proximal left external iliac artery stenosis (white arrow). C, Retrograde access was obtained at both groins and sheaths placed. The left groin sheath totally occludes the left external iliac artery. D, Balloon expandable stents were positioned with proximal ends “kissing” and balloons inflated simultaneously to deploy the stents. A second overlapping stent was inserted in the proximal left external iliac artery. E, Final angiogram shows widely patent vessels after stent insertion.
Predilation with a balloon catheter ensures that the lumen can be fully expanded and that the appropriate balloon and stent size is chosen to avoid vessel rupture. In reality, unless the lesion is heavily calcified, most interventionalists perform primary stent placement to expedite the procedure and theoretically decrease the risk of distal embolization of plaque fragments.
Stents must cover all of the diseased segment and ideally end at a relatively normal point on the artery. Notably, the internal iliac artery often remains patent even with a bare stent placed across its origin. Either self-expanding (SE) or balloon-expandable (BE) stents may be used60,67,68 (see Chapter 3). When precise placement is critical (e.g., at the aortic bifurcation), BE stents are advantageous. When long arterial segments must be covered, SE stents are a wise choice. Although no single device has proven most effective or durable, there is good evidence that nitinol stents yield better long-term results than other materials.
For iliac disease at or just adjacent to the aortic bifurcation, so-called “kissing stents” are often placed. In this technique, bare stents are deployed simultaneously from both groins in both iliac arteries with the superior ends touching in the very distal aorta60,68 (see Fig. 8-24). In this way, overhanging aortic disease is covered and a unilateral stent extending into the aortic lumen will not infringe on the contralateral common iliac artery. However, this common practice may be unnecessary if the opposing iliac vessel is normal and the stent is deposited just up to the bifurcation.69
Table 8-3 summarizes the primary patency rates for endovascular iliac artery treatment.* Eight- to 10-year primary patency rates of iliac artery stents (including occlusions) have been reported at 46% to 74%.70,71 Secondary patency rates at 3 years approach 90% to 95% with most devices. The most commonly reported predictors of long-term durability are male gender, abstinence from smoking, common iliac artery location, large arterial (stent) diameter, patent outflow arteries, short lesion length, and complete covering of the diseased segment.14,70 For example, patency rates for common versus external iliac artery stent placement at 5 years in one study were 76% and 56%, respectively.74 This response may be related to the smaller size of the vessel and the fact that hip flexion causes arterial angulation in this region (not just around the CFA as commonly believed).75
Iliac artery occlusion (TASC type b-d)
Endovascular therapy is an attractive alternative to surgery for some common or external iliac artery occlusions. Balloon angioplasty alone is not sufficient; all of these obstructions require a stent. It is also accepted practice to skip thrombolysis or mechanical thrombectomy and proceed directly to primary uncovered stent insertion once the obstruction is crossed.76
An ipsilateral groin approach provides the most direct pathway to the obstruction. It is also preferable when stents must be deployed up into the aorta. However, many devices can be advanced from the opposite groin over the bifurcation, through a guiding catheter or sheath, and then deployed in the main iliac artery trunks. Rarely, a brachial artery approach is necessary. The occlusion is crossed with a steerable hydrophilic guidewire. If the guidewire will only traverse the occlusion from the contralateral groin, the tip can be snared from the ipsilateral CFA and pulled through the sheath. An angiographic catheter is then placed in a retrograde direction for stent placement. Stents may be inserted even when guidewire passage is partially subintimal, as long as the wire reenters the lumen before reaching the aorta. Stents are deposited along the entire length of the occlusion, incorporating adjacent segments of significant atherosclerotic disease. Placement of stents well into the CFA is controversial (see later discussion).
Revascularization of TASC B and C iliac artery occlusions is somewhat less successful than TASC A iliac stenoses14 (see Table 8-3). However, the difference is largely attributable to cases in which the occlusion cannot be traversed with a guidewire. Thus, following a technically successful procedure, long-term patency rates are about 15% higher than published figures.76–79 The overall complication rate is about 10%. The frequency of distal embolization is about 2% to 5% whether or not thrombolysis is performed.
Internal iliac artery stenosis
Occasionally, proximal internal iliac artery stenoses cause isolated buttock claudication.80–82 Balloon angioplasty and stents have been used successfully to relieve such lesions.
Common femoral artery obstruction
Often, isolated CFA stenoses and occlusions are best treated surgically (e.g., endarterectomy or patch angioplasty), because the operation is relatively simple and is more lasting than angioplasty.83–85 Also, the long-term behavior of metallic stents near this site of constant motion has not been established.
Deep femoral artery stenosis
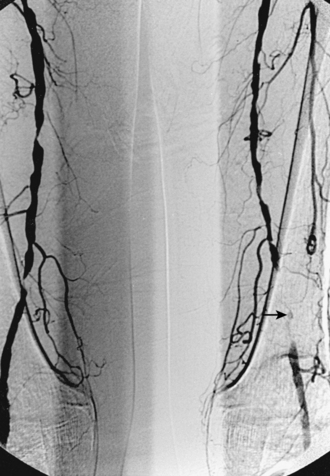
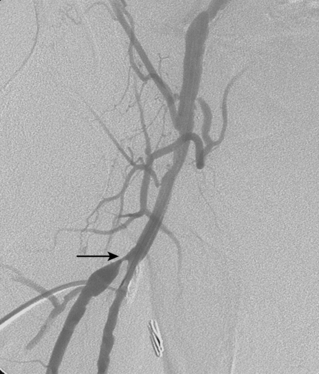
Given the ease and durability of endarterectomy for focal proximal DFA disease, angioplasty or stenting is a second line treatment at this critical site (Fig. 8-25). Reports of long-term clinical success have been sporadic.86–88
Femoropopliteal artery stenosis (TASC types a and b)
Endovascular therapy is first-line treatment for many femoropopliteal lesions in patients with appropriate symptoms14,89,90 (Figs. 8-26 and 8-27). Unlike the iliac artery, the best endovascular approach is often controversial. The outcomes of “plain old” balloon angioplasty and bare stent placement in the femoropopliteal artery have been disappointing compared with applications in the iliac artery (Fig. 8-28). Leading explanations for this disparity invoke (1) the larger atherosclerotic burden of the long femoropopliteal segment, (2) constant extrinsic compression of the SFA at the adductor canal, and (3) hemodynamic stresses related to frequent and significant bending and shortening of the vessel with leg motion. Numerous strategies have been pursued to improve on early modest results.