Chronic pelvic pain is a common and disabling condition affecting adult women. Establishing a specific clinical diagnosis is often difficult. Imaging plays an important role in the diagnosis of urethral diverticula, other periurethral cystic lesions, and pelvic varices. In this chapter a systematic review of the clinical and imaging findings of urethral diverticula, periurethral cystic lesions, and pelvic varices in adult women is presented.
Urethral Diverticulum
Definition
Urethral diverticulum is an epithelialized cavity dissecting within the confines of the fascia of the urethropelvic ligament, and which communicates with the urethral lumen.
Prevalence and Epidemiology
The diagnosis of female urethral diverticulum is a challenging problem for many clinicians, although symptomatic diverticula are now detected with greater frequency owing to awareness of the condition along with the development of sophisticated imaging techniques. The true prevalence of female urethral diverticula is unknown but is likely higher than that reported in 1% to 6% of adult females. Urethral diverticula usually manifest in the third to sixth decades.
Etiology and Pathophysiology
Female urethral diverticulum currently is thought to be an acquired condition and is attributed to the rupture of obstructed and infected periurethral glands into the urethral lumen as a result of weakening of the wall. This cavity or coalesced collection of microabscesses of periurethral glands is then epithelialized and becomes a true diverticulum. This theory is supported by the finding that all urethral diverticula are contained within the periurethral fascia. Another potential cause is disruption of the periurethral fascia during bladder neck suspension surgery for stress incontinence, resulting in focal posterior urethral prolapse. Periurethral diverticula also may develop after obstetric trauma or urethral instrumentation. Congenital female urethral diverticula are rare.
Urethral diverticula are usually located posterolateral to the mid to distal urethra, where most of the periurethral glands are present. A urethral diverticulum frequently has a narrow neck (ostium), which may become occluded. Diverticula may be simple or complex in configuration.
Manifestations of Disease
Clinical Presentation
The classic manifestation of urethral diverticular disease has been described as the three Ds: D ribbling, D yspareunia, and D ysuria. In most patients, however, the signs and symptoms are often nonspecific; therefore an accurate diagnosis is frequently delayed. Postvoid dribbling is present in 25% of patients, and dyspareunia is noted in 10% of patients. The most frequent presenting symptom is urgency and frequency, which occurs in 40% to 100% of patients. Thirty percent to 50% of patients report recurrent urinary tract infections. Other symptoms and signs of urethral diverticular disease include stress and urge urinary incontinence, tender or painful urethral mass, hematuria, and discharge of pus per urethra.
In evaluating urethral diverticula, an anterior vaginal wall mass, which is often tender, may be identified at physical examination, and compression or “milking” of this mass may result in purulent discharge from the urethra. Urethroscopy may reveal the diverticular opening into the urethra, although the openings are often difficult to locate even when the presence of the diverticulum is known. Routine cystoscopy done to evaluate the bladder for nonspecific pelvic discomfort rarely detects a diverticular orifice without previous detection of the diverticulum.
Urethral diverticula may be complicated by active infection (abscess), stone formation (up to 10% of patients), or malignant degeneration. Adenocarcinoma is the most frequently diagnosed tumor in female urethral diverticula.
Imaging Indications and Algorithm
Various imaging techniques are available for establishing the diagnosis of a urethral diverticulum. The diagnosis was traditionally made with voiding cystourethrography and double-balloon urethrography. More recently, magnetic resonance imaging (MRI) with pelvic phased-array coils has become the imaging modality of choice.
Imaging Technique and Findings
Radiography
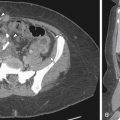
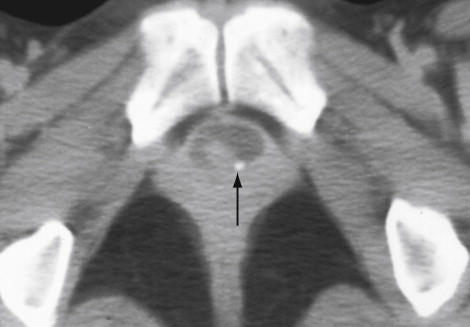
Plain radiographic image of an excretory urogram (intravenous urogram or intravenous pyelogram) may depict occasional stones in a urethral diverticulum. The postvoid image may demonstrate pooling of contrast media underneath the bladder base ( Figure 12-1 ).

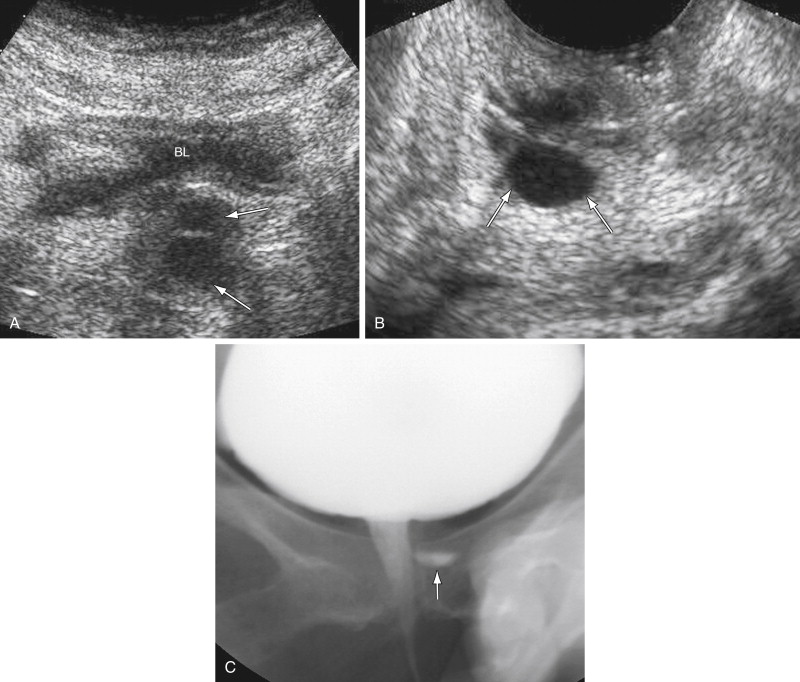
Voiding cystourethrography has an overall accuracy of 85% in detecting urethral diverticula. During the examination, fluoroscopic evaluation of the urethra is performed during active micturition after instillation of contrast material into the bladder via a transurethral catheter. Spot film images are obtained to document a urethral diverticulum communicating with the urethra ( Figure 12-2 ). The neck of the urethral diverticula must be patent to be visualized. The detection of filling defects within the diverticula suggests the presence of urethral calculi, clot, debris, and tumors. The disadvantages of this technique include radiation exposure and transurethral catheterization.
Double-balloon (positive-pressure) retrograde urethrography is more sensitive than voiding cystourethrography and may allow contrast material to be forced into a diverticular ostium by creating a relatively closed urethral system subjected to increased pressure. This technique, however, is difficult to learn, cumbersome, requires exposure to radiation, and may be painful for patients. It is rarely performed.
Ultrasound
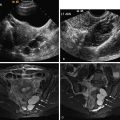
Transabdominal ultrasound (TAUS) using the full urinary bladder as a window can detect large (>2 cm) urethral diverticula (see Figure 12-2 ). Ultrasound (US) is sensitive in differentiating cystic lesions from solid lesions. Transperineal and transvaginal US (TVUS) may detect a smaller urethral diverticulum that does not fill with contrast media and therefore cannot be identified on urethrography. TVUS with a 7- to 10-MHz transducer is more sensitive for detecting urethral diverticula in women compared with transperineal US. The urethra is not seen on a routine TVUS of the pelvis because the transducer is typically inserted past the level of the urethra. TVUS of the urethra requires only partial insertion of the transducer into the vagina.
US can demonstrate a relatively echo-free cavity adjacent to the urethra and may also demonstrate intracavitary debris or surrounding inflammatory edema. The ostium of a diverticulum may be difficult to visualize with US. US advantages include a lack of radiation and lower expense than MRI and computed tomography (CT). The utility of US in making this diagnosis, however, is operator dependent.
High-resolution transurethral US is not readily available and is less convenient because of high equipment expense, patient discomfort, and limited field of view.
Computed Tomography
Urethral diverticula appear as periurethral areas of low attenuation on CT scans. Stones in the urethral diverticulum are readily identifiable on CT ( Figure 12-3 ). When a urethral diverticulum communicates with the urethra, contrast may fill in on delayed enhanced CT scans, particularly after voiding.
CT scans obtained during micturition after instillation of diluted contrast media into the urinary bladder can demonstrate a contrast-filled urethra, which is referred to as CT voiding urethrography . CT voiding urethrogram may reveal a contrast-filled urethral diverticulum if the ostium is patent. Two-dimensional multiplanar and three-dimensional reformatted images can be generated from thin-section CT scans and can permit accurate measurement of diverticulum size. The success of this technique, however, depends on the patient’s cooperation to spontaneously urinate at the exact time of scan acquisition. Another disadvantage is relatively high radiation exposure.
Magnetic Resonance
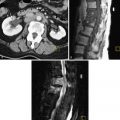
MRI has multiplanar imaging capability, excellent tissue contrast, and lack of ionizing radiation. The normal female urethra has a characteristic target appearance with concentric layers of different signal intensity on axial T2-weighted images. MRI with pelvic phased-array coils is an excellent modality for demonstrating urethral diverticula and for surgical planning purposes ( Figures 12-4 and 12-5 ). MRI is more sensitive than voiding cystourethrography and double-balloon urethrography for the detection of urethral diverticula, including noncommunicating urethral diverticula.

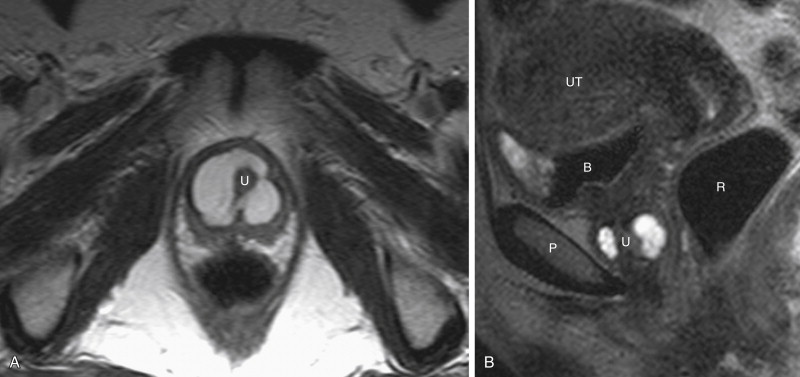
Urethral diverticula are usually located posterolateral to the mid to distal urethra. Diverticula may be simple (single, unilocular) or complex (multiple or multiloculated). Urethral diverticula partially surrounding the urethra are often referred to as horseshoe, saddle, or saddlebag-type diverticula. MRI is optimal at displaying this complex internal diverticular anatomy to assist surgical planning. When a diverticulum originates from the proximal urethra and nearly completely surrounds the urethra, there may be a mass effect on the bladder base in a similar fashion to that seen in elderly men with an enlarged prostate, which is referred to as the “female prostate” sign.
MRI can also depict abnormalities in adjacent structures that could be the cause of the patient’s symptoms. MRI provides excellent visualization of diverticula and their complications and is useful for surgical planning purposes. The use of endovaginal or endorectal coils with MRI can provide higher resolution views of urethral diverticula.
When filling defects are present within the urethral diverticula on MRI, contrast enhancement of that abnormality after intravenous contrast administration indicates a tumor ( Figure 12-6 ). Stones, blood clot, and debris do not enhance after intravenous contrast administration.

Nuclear Medicine and Positron Emission Tomography
Neither nuclear scintigraphy nor positron emission tomography/CT plays a role in the diagnosis of urethral diverticula.
Angiography
Angiography is not helpful in the evaluation of patients with urethral diverticula.
Classic Signs
No classic signs of urethral diverticula have been described in the literature.
Differential Diagnosis
From Clinical Presentation
Because of the nonspecific nature of many patients’ symptoms with urethral diverticula, more common diagnoses such as interstitial cystitis are first considered in many cases. Differential diagnosis of a urethral or vaginal mass includes urethrocele, cystocele, vaginal wall inclusion cyst, ectopic ureterocele, Skene’s gland abscess, Gartner’s duct cyst, primary urethral neoplasms (leiomyoma, carcinoma), and secondary tumors of the urethra with contiguous extension of malignancy from the bladder, uterine cervix, vagina, rectum, and anus.
From Imaging Findings
Periurethral Cystic Lesions
The differential diagnosis of periurethral cystic lesions includes Skene’s gland cyst, Bartholin’s gland cyst, Gartner’s duct cyst, müllerian duct cyst, epidermal clitoral inclusion cyst, cystocele, and postprocedural changes from periurethral injection of collagen or other urethral bulking material.
Skene’s Gland Cysts
Skene’s ducts are embryologically derived from the urogenital sinus. Skene’s ducts are situated in the lamina propria of the lower third of the female urethra, with the ducts opening on the lateral aspect of the external urethral meatus. Cysts of Skene’s gland may be caused by obstruction of the ducts secondary to inflammation. The lesions are often asymptomatic and found incidentally on cross-sectional imaging. A Skene’s gland cyst can become palpable and symptomatic when it becomes complicated with infection. A Skene’s gland cyst is typically located at the posterolateral aspect of the external urethral meatus inferior to the pubic symphysis ( Figure 12-7 ). It may be difficult to differentiate Skene’s gland cysts from Bartholin’s gland cysts. The imaging appearance may become atypical if complicated with intracystic hemorrhage or infection ( Figure 12-8 ). Malignant involvement of the Skene’s gland is rare but reported, with adenocarcinoma the most common cell type. Symptomatic cysts may be treated by excision or marsupialization.