This concept of a Doppler shift is used to measure blood flow velocity whereby the shift in sound-wave frequency is detected by the ultrasound transducer after encountering active blood flow.
However, several factors influence the resultant frequency shift and hence the measured velocity. These include the incident frequency of the ultrasound beam used, speed of sound in soft tissues, the velocity of the moving reflectors (i.e., blood in a vessel), and the angle between the incident beam and vector of blood flow (θ) called the angle of insonation.
The angle of insonation is inversely related to Doppler shift. Hence, as the angle of insonation increases, approaching 90°, the Doppler shift decreases and therefore the calculated blood flow velocity decreases to 0. The Doppler angle is therefore a significant technical consideration in performing duplex Doppler examinations, and an ideal angle of insonance between 0 and 60° is required (Fig. 7.1).
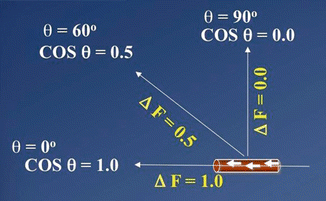

Fig. 7.1
Doppler angle : The change in Doppler frequency (ΔF) is directly related to the cosine of the angle of insonance (θ). The angle of insonance (the angle between the incident beam and the vector of blood flow) must be less than 60° for accurate measurements of blood flow velocity
Clinical Pearl: Even if the angle of insonance is not corrected, the RI will be accurate. However, PSV and EDV will be inaccurate.
Patient Preparation
The patient should lie comfortably on the examination table in a supine position with legs together providing support for the external genitalia. An alternative position is dorsal lithotomy with the penis lying on the anterior abdominal wall. Regardless of the patient position preferred, the area of interest should remain undraped for the duration of the examination. Care should be taken to cover the remainder of the patient as completely as possible including the abdomen, torso, and lower extremities. Ample amounts of ultrasonographic acoustic gel should be used between the transducer probe and the surface of the penis to allow uninterrupted transmission of sound waves, thus producing a high quality image without acoustic interruption.
Penile Ultrasound Protocol
As with other ultrasound exams, penile ultrasound uses specific scanning techniques and images targeting the clinical indication prompting the study. Irrespective of the indication for penile ultrasound, routine scanning during penile ultrasound should include both transverse and longitudinal views of the penis by placing the transducer probe on the dorsal or ventral aspect of the penis. The technique presented here uses a dorsal approach, which is easier for the flaccid phallus. However, the ventral approach, often with placement of legs in the lithotomy position, is often better with a fully erect phallus as well as allowing for better visualization of the proximal corpora cavernosa. The goal is to visualize the cross-sectional view of the two corpora cavernosa dorsally and the corpus spongiosum ventrally along the length of the penis from the base of the penile shaft through to the distal glans penis.
The corpora cavernosa appear dorsally, as two homogeneously hypoechoic circular structures, each surrounded by a thin (usually less than 2 mm) hyperechoic layer representing the tunica albuginea that envelops the corpora. The corpus spongiosum is a ventrally located circular structure with homogeneous echotexture, usually more echogenic than the corpora cavernosa [1]. It is best visualized by placing the ultrasound transducer probe on the ventral aspect of the penis; however, it is easily compressible so minimal pressure should be maintained while scanning. For routine anatomic scanning of the penis with ultrasound, all three corpora can be sufficiently viewed from a single dorsal approach to the penile shaft. A survey scan, with storage of a cine loop of this scan, is first performed prior to obtaining static images at the proximal (base), mid portion, and distal (tip) of the corpora cavernosal bodies for documentation (Figs. 7.2, 7.3, and 7.4). The value of the survey scan cannot be overstated. It often provides the prospective that is necessary to assure absence of coexisting pathology. A careful survey scan of the phallus will identify abnormalities of the cavernosal vessels, calcified plaques, and abnormalities of the spongiosa tissue.
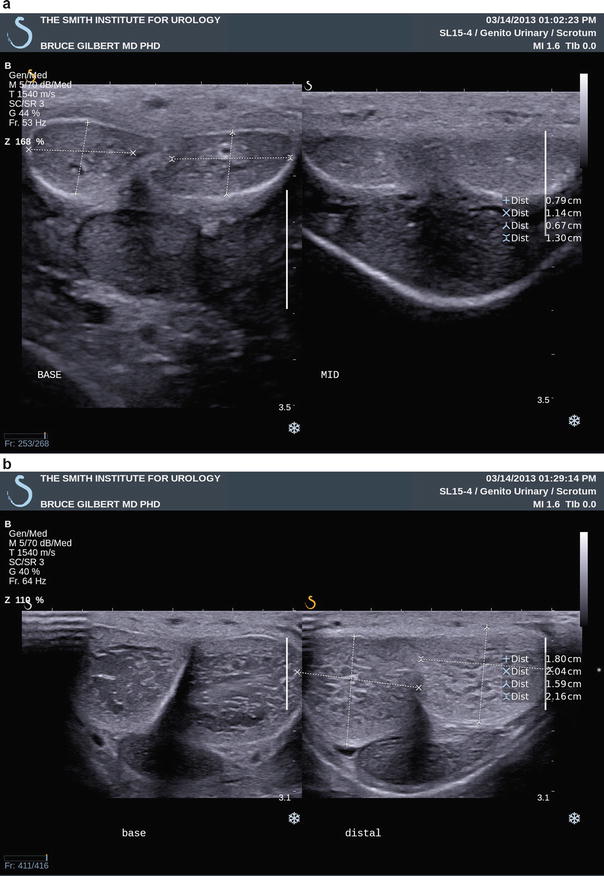
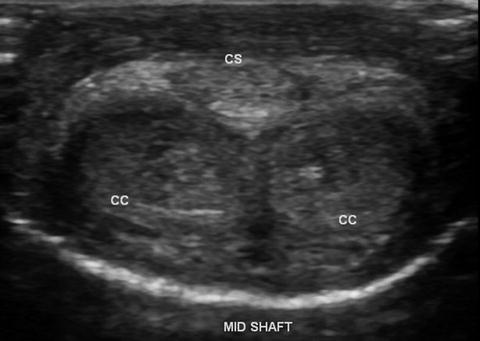
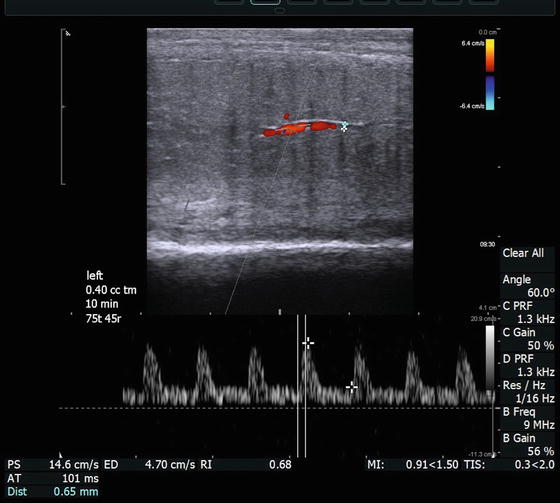

Fig. 7.2
(a) Survey scan with transverse views through the base (left panel) and mid-shaft (right panel) of the penis. In this image the transducer is on the dorsal penile surface and demonstrates the right and left corpora cavernosa nearer the ultrasound probe and corpus spongiosum in the midline ventrally, furthest from the ultrasound probe. (b) Survey scan with transverse views through the base (left panel) and distal shaft (right panel) regions of the penis. Similarly, in this image the transducer is on the dorsal penile surface and demonstrates the right and left corpora cavernosa dorsally (closest to the ultrasound probe) and urethra ventrally (away from the ultrasound probe)

Fig. 7.3
Demonstrates normal mid-shaft view with the ultrasound transducer on the ventral surface of the phallus depicting the right and left corpora cavernosa (CC) and corpus spongiosum (CS)

Fig. 7.4
Longitudinal view of (a) left corpora cavernosa taken 10 min after injection of 0.4 cc of a pharmacologic agent to induce an erection, displaying cavernosal artery diameter, PSV, and EDV
Still images recommended as representative views of this initial surveying scan include one transverse view at the base of the penile shaft, one at the mid-shaft, and a third at the distal shaft just proximal to the corona of the glans penis (Fig. 7.2a, b). Each image should show transverse sections of all three corporal bodies. As noted in the labeled images, orientation by convention is for the right corporal body to be on the left side of the display (as viewed by the sonographer) while the left corporal body is located on the right side of the display on images obtained with the ultrasound probe on the dorsal aspect of the phallus. Figure 7.3 demonstrates a normal mid-shaft view with the transducer on the ventral aspect of the phallus. A longitudinal projection splitting the screen view helps to compare the right and left corporal bodies. Figure 7.4 demonstrates a dorsal approach with measurements of the cavernosal artery diameter, and peak systolic and end diastolic velocities. By convention, the orientation is constant, with the projection of the right corporal body on the left side of the display while the left corporal body is located on the right side of the display.
Embryology Relevant to Ultrasound Imaging of the Penis
A basic understanding of the embryologic development of the genitalia helps guide the interpretation of many abnormalities found on penile ultrasound. Presented in this section is the embryology relevant to the ultrasound evaluation of the penis. The development of the phallus is in close association with the development of the scrotal structures. In addition, the development of the urinary and reproductive system takes place with the simultaneous development of all organ systems from the trilaminar embryo to those systems fully formed at the time of parturition. For a more detailed description of the interplay between these systems, there are several comprehensive texts to which the reader may be referred [2–5].
Development of the Urogenital Sinus
The penis serves as a conduit through which the most distal portion of the urinary tract passes. The lower urinary tract develops as a descendent of the endodermal hindgut, an offshoot that divides from the fledgling gastrointestinal system to interact with the mesonephros and metanephros and ultimately develop into a unique urinary system. In the early embryo, the cloacal membrane is located at the caudal end of the developing hindgut as a bilaminar apposition of ectoderm and endoderm located on the ventral midline (Fig. 7.5). The remainder of the cloaca is a chamber shared by the allantois (which extends anteriorly from the cloaca into the umbilical cord) and the hindgut. The cloacal membrane makes up the ventral wall of the chamber. A septum develops as an ingrowth of folds from the lateral walls and a caudal extension of the intervening mesenchyme from the branch point of the allantois and hindgut, which ultimately divides the cloaca into the anterior/ventral urogenital sinus and the posterior/dorsal developing rectum. While the septum develops, mesodermal mesenchyme also encroaches between the two layers of the cloacal membrane. The septum also divides the membrane into a urogenital membrane and anal membrane. These membranes ultimately rupture to create a continuity between the ectoderm and both the urogenital sinus and rectum. The mesenchymal tissues that have encroached develop into the muscles and bones of the lower anterior abdomen and pubis. An incompletely characterized defect in this process results in the exstrophy-epispadias complex, a spectrum of abnormalities that can include continuities between the luminal surface of the bladder and the lower abdominal skin. If the defect occurs early enough in the process, specifically before the complete division of the cloaca, a cloacal exstrophy can occur which also includes a continuity of the intestinal lumen with the skin and bladder lumen. This is often found on prenatal ultrasound with an appearance that mimics the complete absence of a bladder in the fetus. These findings can also be found with penile anomalies.
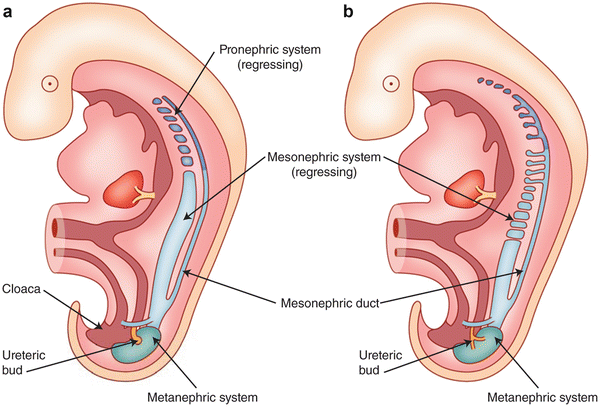

Fig. 7.5
Development of the early excretory system . Regression of the pronephros (a) and mesonephros (b) with the development of the metanephric system
The urethra, prostate, and bladder all develop from the urogenital sinus. The remnants of the caudal ends of the mesonephric ducts become incorporated into the urogenital sinus and become aspects of the trigone and posterior urethra. The incorporated portions of the mesonephric ducts include the branch points of the metanephric ducts, which become the ureteral orifices. The unincorporated portions of the mesonephric ducts end up entering the urethra at the prostatic urethra as the ejaculatory ducts. Ridges in the urethra, called plicae colliculi, remain along the path of the fusion of the mesonephric ducts as they become incorporated with the urogenital sinus and migrate while the sinus develops. It is hypothesized that abnormalities in this process result in the fusion of the plicae colliculi that is seen in the majority of posterior urethral valves. The remainder of posterior urethral valves is thought to result from an incomplete rupture of the urogenital membrane. Posterior urethral valves are associated with a dilated posterior urethra (Fig. 7.6) and thickened bladder wall on ultrasound, referred to as the keyhole sign. The bladder is often distended with marked hydroureteronephrosis also demonstrated on ultrasound examination.
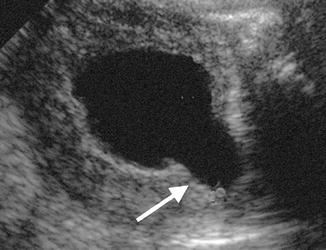

Fig. 7.6
Ultrasound of the bladder with the typical findings associated with posterior urethral valves including a thickened wall and dilated posterior urethra known as the keyhole sign
Development of the Male External Genitalia and Phallus
Up until the eighth or ninth week of gestational age the development of the external male genitalia is indistinguishable with that of the female genitalia. It is characterized in both genders by the development of the genital tubercles at the craniolateral edges of cloacal membrane. They develop from mesoderm as it infiltrates the cloacal membrane. As the cloaca membrane divides with the anterior portion becoming the urogenital membrane, the two tubercles fuse in the midline. This fusion can be disrupted and results in the bifid phallus that is sometimes seen in conjunction with bladder exstrophy. The urogenital membranes and ultimately the urogenital sinus are flanked by collections of infiltrating mesoderm termed the urogenital folds with labial scrotal swellings located laterally either side (Fig. 7.7). Masculinization of the indifferent external genitalia occurs under the influence of testosterone produced by the interstitial cells of the fetal testis [6–8]. The tubercle becomes the future phallus and glans. The terminal part of the phallus destined to be the glans becomes solid. The groove between the urogenital folds, which extend onto the underside and with the tubercle as the tubercle grows, is termed the urogenital groove. The urogenital sinus extends to the undersurface of the genital tubercle where it opens in this groove. Apical ridges of the urogenital folds grow towards each other and the walls of the phallic portion come together and fuse which creates a tubular extension of the urogenital sinus on the caudal aspect of the developing phallus. This opening is for a while the primitive urogenital opening, and it extends forward to the corona glandis (Fig. 7.7) and the final urethral meatus. Abnormalities in this process can result in the ectopic location of the urethral meatus along the ventral phallus or midline scrotum, penoscrotal junction, or scrotoperineal junction, termed hypospadias. This is often associated with a hypoplastic development of the corpus spongiosum which can be demonstrated ultrasonographically.
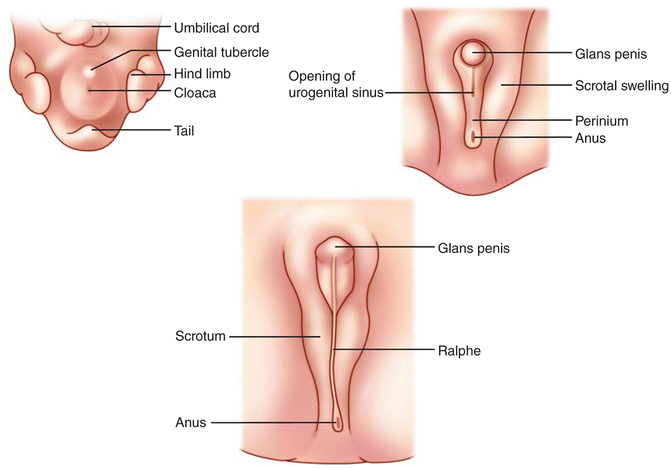

Fig. 7.7
The male external genital development progressing clockwise from the left. The genital tubercle develops into the glans penis. The upper right panel shows the urogenital sinus opening within the urogenital folds, which are between the scrotal swellings
The corpora cavernosa of the penis as well as the spongiosum surrounding the urethra arises from the mesodermal tissue in the phallus. They are at first dense structures, but later vascular spaces appear in them, and they gradually become cavernous. A solid plate of ectoderm grows over the superficial part of the phallus. A bilayer tissue is formed by a breakdown of the more centrally situated cells forming the prepuce. The scrotum is formed by extension of the labioscrotal swellings between the pelvic portion and the anus. With testicular decent these labioscrotal swellings form the scrotal sacs (Fig. 7.7).
Penile Blood Supply
The anterior trunk of the internal iliac gives rise to the internal pudendal artery approximately at the level of the greater sciatic foramen (Fig. 7.8a and white circle in Fig. 7.8b). The internal pudendal artery then crosses behind the tip of the ischial spine and enters the peritoneum through the lesser sciatic foramen (Fig. 7.8b). It then enters then passes through Alcock’s canal in the ischiorectal fossa to become the penile artery. It is at this point that the internal pudendal artery is most susceptible to injury (yellow circle in Fig. 7.8b). The penile artery passes through the urogenital diaphragm and along the medial aspect of the inferior limits of the pubic symphysis (Fig. 7.8c).
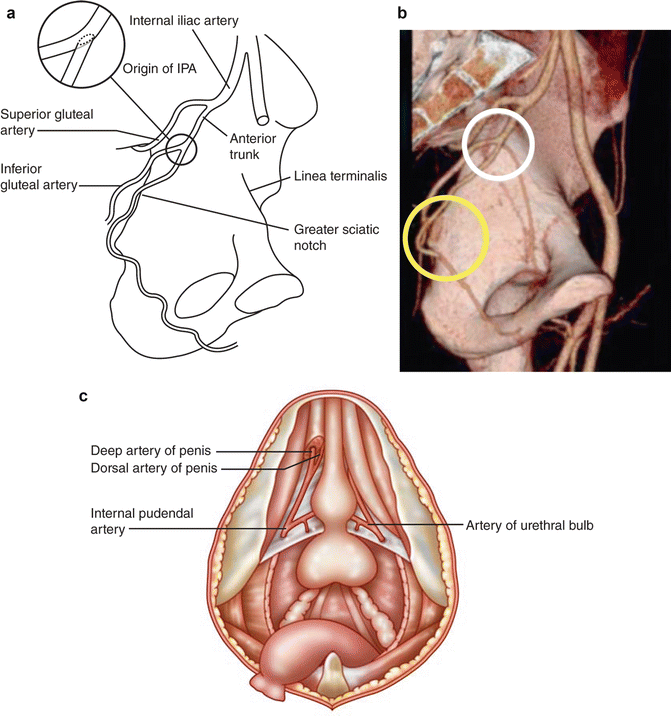

Fig. 7.8
Arterial blood supply to the penis and path through the pelvis in labeled schematic (a), in 3D imaging reconstruction (b). The white circle highlights to origin of the internal pudendal artery. The yellow circle highlights the path around the ischial spine and into Alcock’s canal. The distal internal pudendal artery gives rise to the artery of the bulb (bulb-urethral artery) and the penile artery (c)
The penile artery then gives rise to branches including the bilateral urethral artery and another dorsal branch which gives rise in turn to the dorsal artery of the penis and the cavernosal artery (Fig. 7.9). The urethral artery travels within the spongiosal tissue and supplies the corpus spongiosa, urethra, and glans penis. The dorsal artery runs deep to Buck’s fascia and just medial to the paired dorsal nerves and lateral to the single deep dorsal vein. The dorsolateral vessel gives rise to circumflex branches that pass around the corpus cavernosum and spongiosum. The cavernosal artery begins at the base of the penis and runs centrally through the corpus cavernosus. The cavernosal artery gives rise to two major branches one that supplies the smooth muscle and nerve fibers of the cavernosal trabecular tissue and the other which divides into a series of helicine arteries. The helicine arteries are unique in that they allow blood to pass directly into the cavernous sinusoids without first traversing a capillary bed. They also allow elongation and dilation of the penis without compromising the blood supply to the corpora [9].
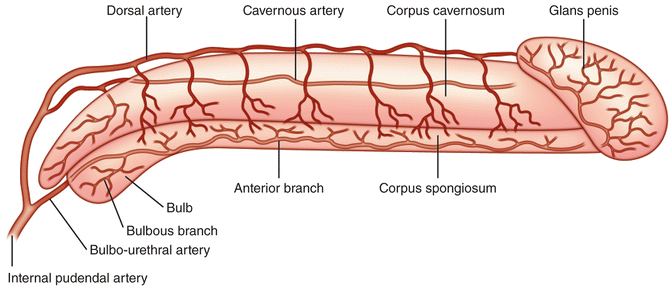

Fig. 7.9
Arterial supply within the penis . The distal internal pudendal artery gives rise to the artery of the bulb (bulbo-urethral artery) and the penile artery which in turn branches into the dorsal and cavernosal arteries of the penis
There are three major venous drainage pathways in the penis [10, 11]. First the superficial receives drainage from the penile skin and layers superficial to Buck’s fascia usually through a single vessel, the superficial dorsal vein, running the length of the dorsal aspect of the phallus. Secondly, the intermediate system deep to Buck’s fascia and superficial to the tunica albuginea receives drainage from the glans, corpus spongiosum, and corpus cavernosum. Emissary veins, primarily from the dorsal and lateral corpora cavernosum , pierce the tunica albuginea and combine to become circumflex veins which enter into the deep dorsal vein. This usually single vein empties into the periprostatic plexus of Santorini. The third major venous drainage pathway is via a deep system that drains the proximal portion of the corpus spongiosum and a major portion of the corpora cavernosum. This drainage travels through the deep penile veins and exits through the pudendal plexus.
The location of penile vessels on ultrasound is dependent upon where the ultrasound probe is positioned on the phallus. Either the dorsal or ventral approach can be used. However, if the dorsal approach is used, the urethra is on opposite side of the cavernosal bodies as the transducer and if the ventral approach is used the urethra is between the transducer and the corpora cavernosa (Fig. 7.10). Note the corpora and urethral are usually more compressed in the ventral approach and therefore visualization of vessels is more difficult (Fig. 7.11). In addition, when the transducer is placed dorsally, the superficial and deep dorsal veins are found near the transducer. However, when the transducer is placed on the ventral aspect of the phallus, the urethral blood supply is closest to the transducer (Fig. 7.11).
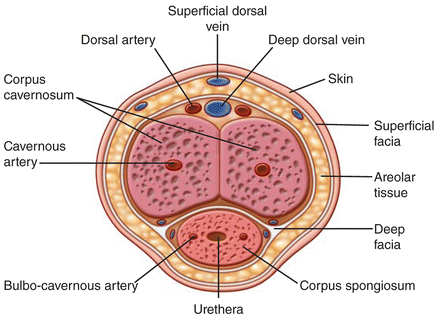


Fig. 7.10
Schematic of transverse view through penis with the distribution of major vessels. This view duplicates the transverse ultrasound view

Fig. 7.11
Transverse B-mode ultrasound views of the phallus with the transducer on the dorsal and ventral aspects. CC corpus cavernosus, RT right, and LT left
The development of the male genitalia is a complex process. The understanding of this process and the abnormalities that correlate to embryologic processes and vestiges are crucial to aid the sonographer in identifying findings on the ultrasound evaluation of the phallus, scrotum, and male reproductive structures.
Focused Penile Ultrasound by Indication
There are several accepted indications for penile ultrasound, each with specialized focus beyond the routine survey scan as previously described. General guidelines for the use of penile ultrasound are delineated by the “Consensus Statement of Urologic Ultrasound Utilization” put forth by the American Urologic Association [12] and the American Institute for Ultrasound in Medicine (AIUM). These indications can be further classified as either vascular, structural, or urethral pathology in nature (Table 7.1).
Table 7.1
Indications for penile and urethral ultrasound
Vascular pathology: |
Erectile dysfunction (ED) |
Cavernosal artery diameter |
Flow velocity |
Peak systolic velocity (PSV) |
End diastolic velocity (EDV) |
Resistive index (Ri) |
Priapism |
High-flow (arterial) |
Low-flow (ischemic) |
Penile trauma/fracture |
Dorsal vein thrombosis |
Structural pathology: |
Penile fibrosis/Peyronie’s disease |
Plaque assessment (number, location, echogenicity, and size) |
Perfusion abnormalities |
Perfusion surrounding plaques |
Penile mass |
Primary penile tumors |
Metastatic lesions to the penis |
Penile foreign body (size, location, echogenicity) |
Penile urethral disease: |
Urethral stricture (location, size) |
Perfusion surrounding plaques |
Calculus/foreign body |
Urethral diverticulum/cyst/abscess |
Erectile Dysfunction
PDU is an integral part of the assessment of patients with ED. Often practitioners use intracavernosal injection therapy with vasoactive agents in patients who have failed a course of oral phosphodiesterase-5 inhibitors. In this situation, PDU may be used as a diagnostic tool in conjunction with commencement of injection therapy. PDU allows for a baseline evaluation of the functional anatomy as well as providing a real-time assessment of the dynamic changes experienced in response to the dosing of vasoactive medications. In cases where intracavernosal injection of vasoactive substances does not prompt a penile erection, documentation provided by PDU will be a foundation for other management options including use of vacuum constriction devices or insertion of a penile prosthesis.
Possibly one of the most compelling reasons for the performance PDU in men presenting with ED is the finding that impaired penile vascular dynamics, as documented on PDU, may be associated with a generalized vessel disease that often predates cardiovascular disease by 5–10 years [13–15]. Significantly, early treatment of metabolic factors (e.g., hypertension, dyslipidemia, hyperglycemia) can delay and possibly prevent the development of cardiovascular disease [16, 17]. Therefore, the physician evaluating ED has a unique opportunity to diagnose vascular impairment at a time when lifestyle changes and possible medical intervention have the potential to change morbidity and mortality of cardiovascular disease. As suggested by Miner, there might be a “window of curability” which we like to refer to as a “window of opportunity” in which the significant risk of future cardiovascular events might be averted through early diagnosis and treatment [18–20].
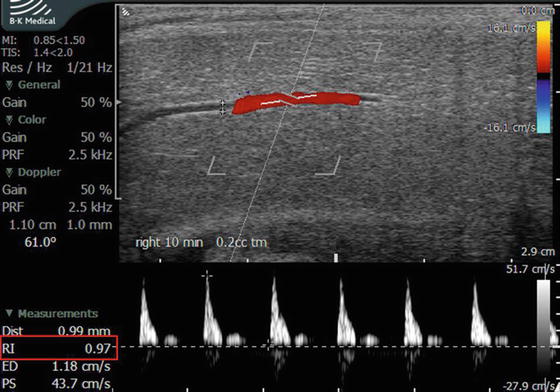
In cases of diagnostic study for ED, emphasis is directed toward the cavernosal arteries. However, the initial survey scan is essential to evaluate for plaques, intracavernosal lesions, and urethral pathology as well as evaluation of the dorsal penile vessels. The cavernosal arteries are visualized within the corpora cavernosa, and the depth of these arteries can be easily defined within the corpora during transverse scanning to ensure a comprehensively represented assessment of diameter at different points along its course. Color Doppler examination of the penis should be performed in both transverse and longitudinal planes of view. Using the transverse views as a guide to cavernosal artery depth, turning the transducer probe 90° then provides longitudinal views of each corpus cavernosum separately, allowing for identification of the cavernosal arteries in longitudinal section (Fig. 7.4). The diameter of the cavernosal artery should be measured on each side. Color flow Doppler makes recognition of the location and direction of blood flow easy. Measurements of vessel diameter to assess the peak systolic flow velocity (PSV) as well as end diastolic flow velocity (EDV) allow for the assessment of a vascular resistive index (RI) (Fig. 7.12). The diameter of the cavernosal artery ranges from 0.2 to 1.0 mm in a flaccid penis [21, 22]. PSV varies at different points along the length of the cavernosal artery, typically with higher velocities occurs more proximally [23]. Hence, assessment of the PSV and EDV should be recorded at the junction of the proximal one-third and the distal two-thirds of the penile shaft. In the flaccid state, cavernosal artery PSV normally measures 5–15 cm/s, at baseline. This should be assessed and compared to the pharmacostimulated state [24, 25].
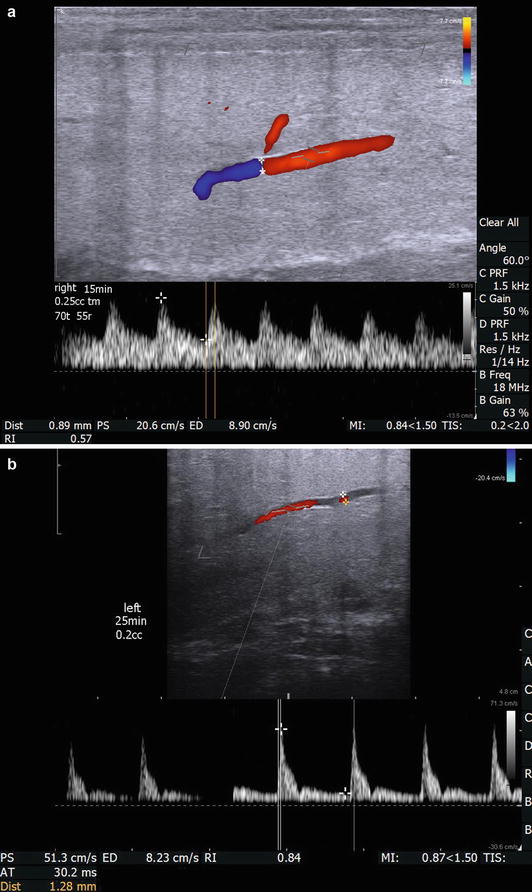

Fig. 7.12
(a) The right cavernosal artery is imaged 15 min after intracavernosal injection of 0.25 mL of trimix solution. The measured vessel diameter is 0.89 mm. The direction of flow and a dorsal branch of the cavernosal artery are easily appreciated with color Doppler. Also, documented on this image is measurement of arterial diameter (0.89 mm), PSV (20.6 cm/s), EDV (8.9 cm/s), and calculated RI (0.57) are shown. Please note that the angle of incidence is electronically made to be 60° by both electronic steering of the transducer and aligning the cursor to be parallel to the flow of blood through the artery. In addition the width of the caliper is adjusted to be approximately ¾ the width of the artery for best sampling. (b) The right cavernosal artery of another patient was imaged at 25 min after intracavernosal injection of 0.2 mL of trimix solution with a measured vascular diameter of 1.28 mm, PSV of 51.3 cm/s, EDV of 8.23 cm/s, and calculated RI of 0.84
The intracavernosal injection should then be given (Appendix 2). At regimented serial time points following the injection of vasoactive medication, cavernosal artery dimensions and flow velocities should be recorded to assess the response to pharmacologic stimulation. After prepping the lateral aspect of the penile shaft with an alcohol or providone-iodine prep pad, a finely measured volume of a vasoactive agent should be injected into one corpus cavernosum (in the distal two-thirds of the penile shaft) using a 29 or 30 gauge ½″ needle. Pressure should be held on the injection site for at least 2 min to prevent hematoma formation.
Vasoactive agents used for pharmacologic stimulation of erection include prostaglandin E1, papaverine, or trimix (combination of prostaglandin E1, papaverine, and phentolamine) [26]. As with every medication administration, the expiration date of the medication should be reviewed, patient allergies should be evaluated, and the dosage administered should be documented. We obtain an informed consent after the patient is counseled about the known risk for developing a low-flow priapism and appropriate follow-up if this were to arise [27]. This protocol requires the patient to stay in the office until penile detumescence occurs. A treatment protocol for low-flow priapism is given in Table 7.2. Of note, for patients in which we have given a vasoactive agent and have had to treat for low-flow priapism, aspiration, irrigation, and injection of intracorporal phenylephrine are usually successful to reverse the priapism state. In our experience, when required, corporal aspiration alone has been uniformly successful in the setting of pharmacologically induced priapism following diagnostic duplex penile ultrasonography.
Table 7.2
Treatment protocol for low-flow priapism caused by pharmacologic induction by vasoactive agents
1. Observation: if no detumescence in 1 h, then |
2. Aspiration: with a 19 or 21 gauge butterfly needle aspirate 30–60 cc corporal blood. A sample should be sent for diagnostic cavernosal blood gas to confirm low-flow, ischemic state. Repeat in ½ h if 100 % rigidity returns |
3. Pharmacologic detumescence: |
(a) Phenylephrine 100–500 mcg injected in a volume of 0.3–1 cc every 3–5 min for a maximum of 1 h |
(b) Monitor for acute hypertension, headache, reflex bradycardia, tachycardia, palpitations, and cardiac arrhythmiã |
(c) Serial non-invasive blood pressure and continuous electrocardiogram monitoring are recommended |
Arteriogenic ED is a form of peripheral vascular disease, commonly associated with diabetes mellitus and/or coronary artery disease . PSV is the most accurate measure of arterial disease as the cause of ED. The average PSV after intracavernosal injection of vasoactive agents in healthy volunteers without ED ranges from 35 to 47 cm/s, with a PSV of 35 cm/s or greater signifying arterial sufficiency following pharmacostimulation [28–33]. Primary criteria for arteriogenic ED include a PSV less than 25 cm/s, cavernosal artery dilation less than 75 %, and acceleration time >110 ms. In cases of equivocal PSV measurements, particularly when PSV is between 25 and 35 cm/s, one should assess for asymmetry of greater than 10 cm/s in PSV between the two cavernosal arteries, focal stenosis of the cavernosal artery, and possible cavernosal artery to cavernosal-spongiosal flow reversal [34].
Veno-occlusive insufficiency , also referred to as venous leak, can only be diagnosed in cases of ED where the patient was confirmed to have appropriate arterial function as measured by PSV. PDU parameters to assess the presence of veno-occlusive insufficiency as the cause of ED are EDV and RI. Antegrade EDV greater than 5 cm/s in the cavernosal artery demonstrated throughout the study, especially at the most turgid level of erection achieved, is suggestive of a venous leak [35, 36]. This is only true if PSV is normal. Arteriogenic dysfunction by definition fails to produce a fully tumescent and rigid phallus. In the setting of venous leak, EDV is always greater than 0. The definitive test for venous leak is the DICC (dynamic infusion cavernosography and cavernosometry). However, when both arteriogenic and venogenic dysfunction exists, interpretation of DICC is difficult. On PDU an RI of less than 0.75, measured 20 min following maximal pharmacostimulation, has been found to be associated with a venous leak in 95 % of patients [37]. In the absence of a venous leak, a fully erect penis should have an EDV nearing zero and hence the RI should approach or exceed (when reverse flow occurs) 1.0 (Fig. 7.13). In cases of diagnostic PDU with intracavernosal pharmacostimulation where a RI of 1.0 or greater is achieved, we recommend immediate treatment or prolonged observation to achieve detumescence because of the high specificity of absent diastolic flow for priapism [38].