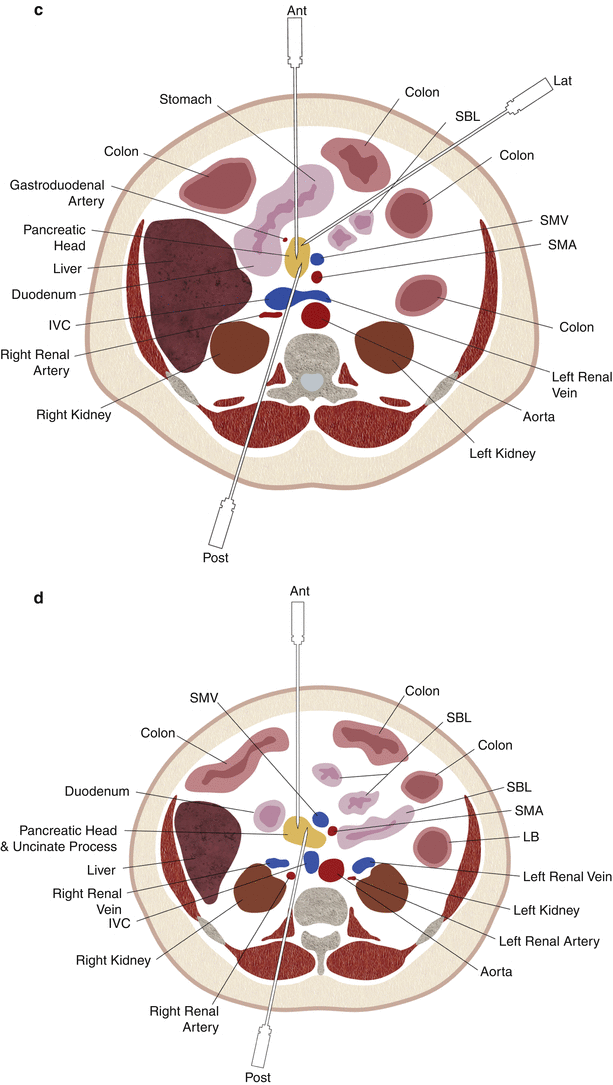
Fig. 14.1
Schematic drawings showing the relevant anatomy and needle trajectories for anterior (Ant), posterior (Post), and lateral (Lat) approaches for percutaneous pancreatic biopsy at various levels: upper pancreatic body and tail (a), lower pancreatic body and tail (b), pancreatic head (c), and uncinate process (d). The needle has been advanced via a posterior approach and traverses the IVC in c. IVC inferior vena cava, SMA superior mesenteric artery, SMV superior mesenteric vein, SBL small bowel loops
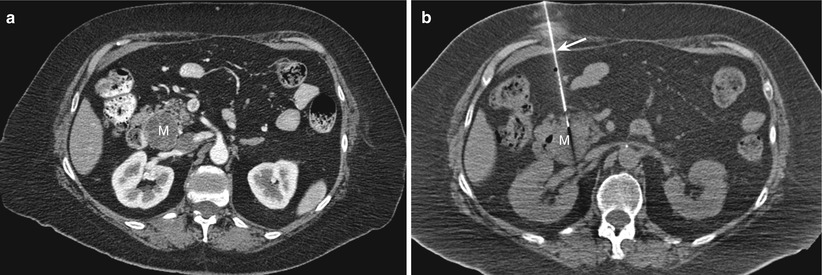
Fig. 14.2
Direct anterior approach. (a) Contrast-enhanced transverse CT scan obtained with the patient in a supine position shows a hypoenhancing mass (M) in the head of the pancreas. (b) Transverse CT scan during the biopsy procedure shows the biopsy needle (arrow) advanced via an anterior approach into the pancreatic mass (M)
The incidence of bowel traversal during ultrasound-guided biopsies reported by Brandt et al. [2] may be an underestimation, because the inadvertent traversal of bowel loops while they are being compressed by the ultrasound probe (a maneuver commonly used during ultrasound-guided biopsy procedures to improve the visualization of lesions in deep abdominal structures) is not uncommon. It is generally acceptable to traverse the gastrointestinal tract in an immunocompetent patient when performing FNABs of abdominal masses [2, 30]. In the study by Brandt et al. [2], none of the patients had complications related to biopsy route. Many other studies have also shown that traversal of the stomach (Fig. 14.3) or small bowel (Fig. 14.4) during percutaneous pancreatic biopsy is unlikely to result in substantial complications [2, 30–32]. Although some authors state that traversing the colon is acceptable during pancreatic biopsies, others avoid the colon, especially when using large-bore needles [5]. Injection of physiologic saline solution through the guide needle can help displace the colon or small bowel loops from the intended needle path and thereby decrease the risks associated with the procedure [33, 34]. Alternatively, using a Hawkins-Akins (Meditech, Westwood, MS) needle with a blunt trocar may also reduce the risk of injury to the bowel and/or major vessels in the needle path [31] (Fig. 14.5). When necessary, an out-of-plane route can be taken to avoid injury to the stomach or bowel.
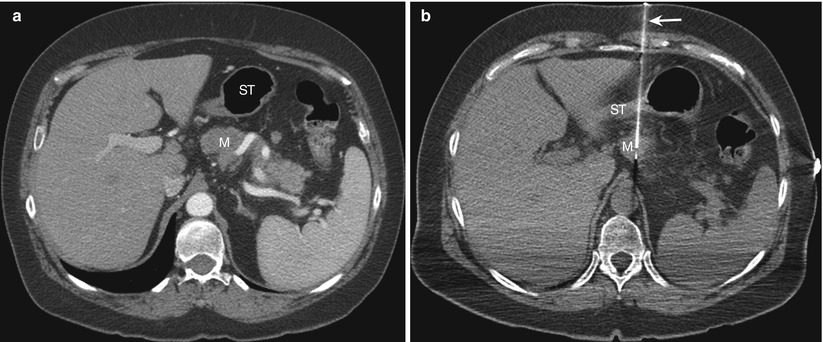
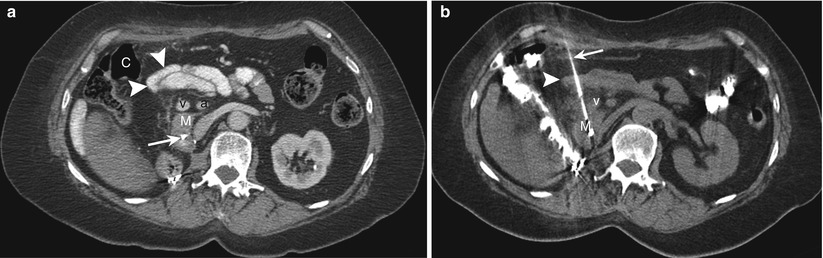
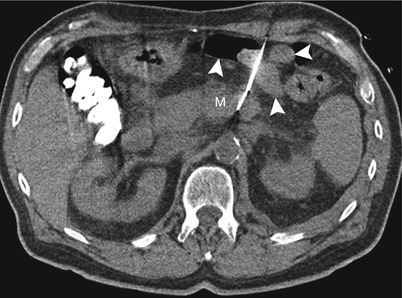

Fig. 14.3
Transgastric approach. (a) Contrast-enhanced transverse CT scan obtained with the patient in a supine position shows a pancreatic mass (M) posterior to the stomach (ST). (b) Transverse CT scan during the biopsy procedure shows the biopsy needle (arrow) advanced through the collapsed stomach (ST) into the pancreatic mass (M)

Fig. 14.4
Needle traversing small bowel loops. (a) Contrast-enhanced transverse CT scan obtained with the patient in a supine position shows a mass (M) in the head of pancreas. A direct anterior approach is precluded by the presence of the hepatic flexure of the colon (C), small bowel loops (arrowheads), and superior mesenteric vein (v) and artery (a). Note the presence of a biliary stent (arrow). (b) Transverse CT scan during the biopsy procedure shows the biopsy needle (arrow) advanced through the small bowel loops (arrowheads) and medial to the superior mesenteric vein (v) into the pancreatic mass (M)

Fig. 14.5
Use of a Hawkins needle. Transverse CT scan during the biopsy procedure shows a Hawkins biopsy needle advanced in between multiple bowel loops (arrowheads) into a pancreatic mass (M)
A transhepatic approach can be used for needle biopsies of lesions in the pancreatic head. Occasionally, an anterior approach to lesions of the pancreatic body may require needle placement through the left lobe of the liver (Fig. 14.6). Needle biopsy through the liver is generally considered safe; however, there is a small risk of needle-track seeding with malignant cells [31].
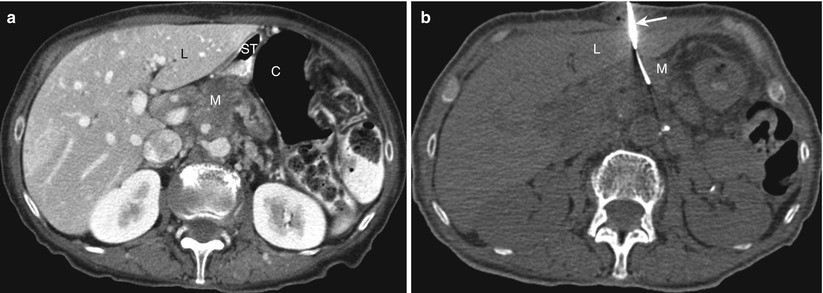

Fig. 14.6
Transhepatic approach. (a) Contrast-enhanced transverse CT scan obtained with the patient in a supine position shows a mass (M) in the pancreatic body. A direct anterior approach is precluded by the interposed liver (L), stomach (ST), and colon (C). (b) Transverse CT scan during the biopsy procedure shows the biopsy needle (arrow) advanced through the left lobe of the liver (L) into a pancreatic mass (M)
If a safe anterior approach is not available, a posterior approach can be used. In our experience, a posterior approach is less painful to the patient because it avoids a transperitoneal puncture. A direct posterior approach from the left side can occasionally be used for lesions involving the distal body or tail of the pancreas (Fig. 14.7). With this approach, care should be taken to avoid injury to the splenic artery and vein, left adrenal gland, upper pole of the left kidney, and spleen. This approach may be precluded by the presence of interposed lung and pleura. The triangulation method, which involves a caudal-skin entry and uses the Pythagorean theorem to calculate the needle angle, or angling of the CT gantry can be used to allow an angled posterior approach that avoids pleural transgression in biopsy of pancreatic tail lesions [35]. Electromagnetic navigation systems can also be used to assist in such out-of-plane approaches [36, 37] (Fig. 14.8). Alternatively, placing the patient in the ipsilateral decubitus position may cause the diaphragm to move superiorly, allowing for a direct posterior approach (Fig. 14.9).
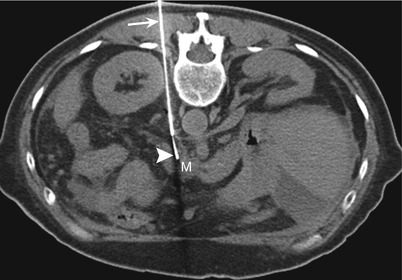
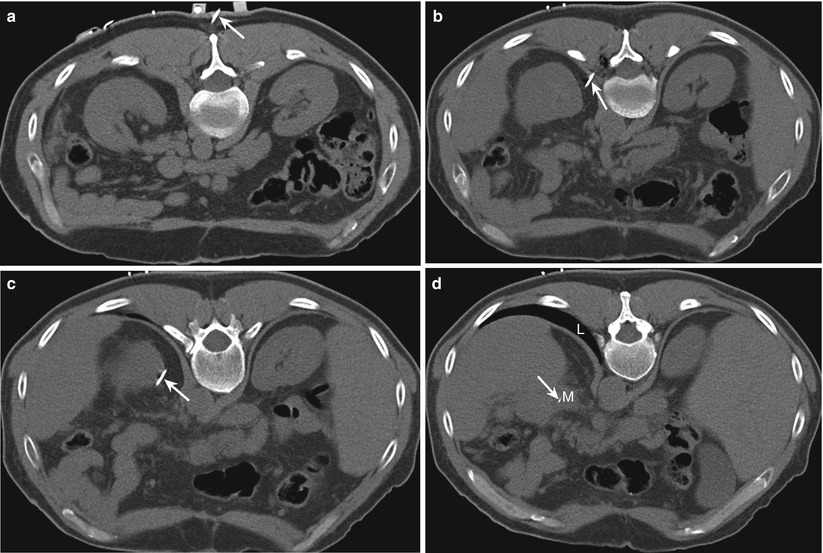
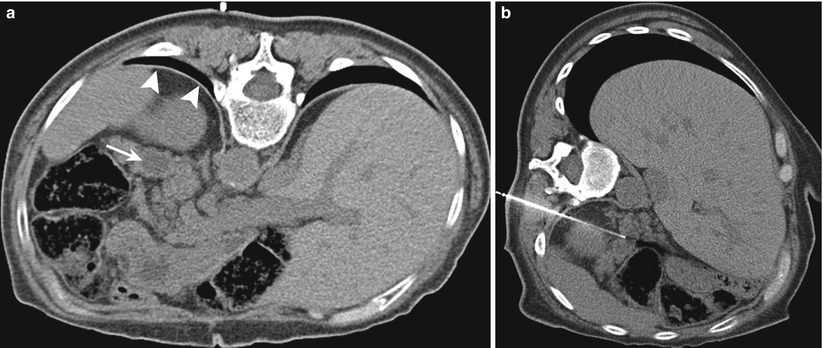

Fig. 14.7
Direct posterior approach. Transverse CT scan with the patient in a prone position shows an 18-gauge guide needle (arrow) advanced through the paravertebral space. A 22-gauge needle (arrowhead) was advanced through the guide needle to sample a mass (M) involving the distal body of the pancreas

Fig. 14.8
Use of electromagnetic navigation system for an angled caudocranial approach. Transverse CT scans from caudal to cranial levels with the patient in a prone position show an 18-gauge guide needle (arrow) inserted at a level caudal to the mass (a) and directed cranially (b–d) to sample a mass (M) involving the tail of the pancreas. A direct approach would have involved a needle passing through the interposed lung (L). The needle was advanced to the lesion in a single pass using virtual real-time multiplanar reconstructions to guide the needle trajectory

Fig. 14.9
Use of an ipsilateral decubitus position to avoid the interposed lung. (a) Transverse CT scan with the patient in a prone position shows the interposed lung (arrowheads) obstructing the direct path to a pancreatic tail lesion (arrow). (b) CT scan with the patient in an ipsilateral decubitus position causes the diaphragm to move superiorly, allowing for a direct posterior approach
While one is using a posterior approach to lesions involving the pancreatic head and uncinate process, the needle is inserted through the right paraspinal muscles and is advanced lateral to the inferior vena cava (IVC); between the IVC and the vertebra (Fig. 14.10); or, if neither route is feasible, through the IVC itself (Fig. 14.11). For the transcaval approach, an 18-gauge guide needle is positioned posterior to the cava IVC, and an inner 22-gauge Chiba needle traverses the vena cava IVC to biopsy the target lesion [7, 38]. Studies have shown that the transgression of vascular structures, especially low-pressure veins, with small-caliber needles does not increase the complication rates of needle biopsy procedures [7, 39]. Two studies describing the use of the transcaval approach for pancreatic biopsies did not report any major hemorrhagic complications [7, 38]. However, when the transcaval approach is used, it is important to identify and avoid the right renal artery, which courses posterior to the IVC.
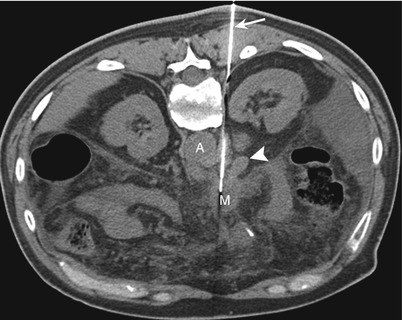
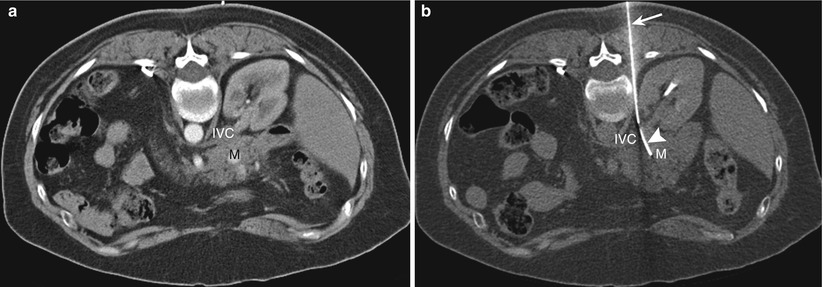

Fig. 14.10
Direct posterior approach. Transverse CT scan with the patient in a prone position shows the biopsy needle (arrow) advanced through the paravertebral space to sample a mass (M) involving the head of the pancreas. The needle passes between the inferior vena cava (arrowhead) and the aorta (A)

Fig. 14.11
Posterior transcaval approach. (a) Contrast-enhanced transverse CT scan obtained with the patient in a prone position shows a mass (M) in the head of the pancreas. Note the presence of the inferior vena cava (IVC) posterior to the mass. (b) Transverse CT scan during the biopsy procedure with the patient in a prone position shows the 18-gauge needle (arrow) posterior to the IVC and the curved 22-gauge needle (arrowhead) traversing the IVC to sample the mass (M)
A direct lateral or anterolateral approach can also be used for needle biopsy of lesions involving the distal body and tail of the pancreas (Fig. 14.1). The structures that may be present in the needle path with this approach include the small and large bowel, spleen, and mesenteric vessels. Saline injection to displace bowel loops or use of a blunt-tip Hawkins-Akins (Meditech) needle can help avoid injury to the bowel.
When pancreatic cancer presents as an isolated cuff of tumor surrounding the superior mesenteric artery or celiac trunk, without an identifiable mass [40], a posterior needle biopsy approach under CT guidance can be used. Administration of a contrast medium may be required to identify and avoid major blood vessels [41]. Particular attention must be used to identify and avoid the superior mesenteric artery; the celiac trunk; and the splenic, superior mesenteric, and portal veins. The needle is inserted and advanced via a posterior-oblique approach lateral to these vessels. Some authors have described the use of an anterior approach with real-time ultrasound guidance for biopsying such lesions; a combination of gray-scale ultrasonography and color-Doppler imaging helps to avoid inadvertent transgression of the artery [41].
Most ultrasound-guided pancreatic biopsies are performed using a single-needle technique that involves using a new needle for each pass. On the other hand, CT-guided pancreatic biopsies generally involve the use of a coaxial technique that requires initial placement of a thin-walled guide needle in or close to the target lesion followed by advancement of the biopsy needle through the guide needle to obtain tissue samples. The coaxial technique allows multiple samples of tissue to be obtained without the need for additional passes through overlying tissues, thus decreasing the risk of complications and patient discomfort. Unlike the single-needle technique, the coaxial technique requires the precise placement of only the guide needle, thus substantially decreasing the duration of the procedure and the number of images required, especially for deep lesions with difficult access routes. Also, the coaxial technique allows for small-caliber CNBs to be performed through the same guide needle. One potential problem with the coaxial technique is that after the first-needle pass, subsequent passes tend to follow the same path and may yield little additional tissue. This problem can be overcome by the use of a custom-made curved 22-gauge needle, which is advanced through a straight 18-gauge guide needle to sample different parts of the lesion, potentially increasing the diagnostic yield [26]. The needle is customized by grasping its tip with a forceps and gently bending the needle so that the distal part of its shaft curves. The curved-needle technique can also help avoid intervening structures or correct for an inaccurately positioned guide needle.
Results
The reported accuracy of image-guided percutaneous biopsy for assessing pancreatic lesions ranges from 45 to 100 % (2). The reported accuracy of CT-guided pancreatic biopsy ranges from 50 to 94 % [2, 42], whereas that of ultrasound-guided biopsy ranges from 67 to 97 % [2, 3, 8, 43]. The wide variability in accuracy reported in the literature may be related to a variety of factors, such as lesion size and location, needle size, biopsy device, and the experience level of the individual performing the biopsy.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree