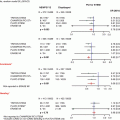
Fig. 28.1
The mitral valve apparatus
Mitral Regurgitation: Etiology, Prevalence, and Clinical Background
The etiologies of MR are multiple and have been described by Carpentier, Barlow, and others (Fig. 28.2) [6–8]. Degenerative mitral regurgitation (DMR, Carpentier Type II dysfunction) is the result of structurally abnormal leaflets or chordae and occurs in fibroelastic deficiency with leaflet prolapse, myxomatous leaflet degeneration, or Barlow’s disease with diffuse excess tissue and chordal elongation. The 10-year natural history of severe MR due to a flail posterior leaflet is known, with 90 % of patients expiring or requiring an MV operation [9]. Surgical MV repair has traditionally been the preferred treatment for degenerative MR, since it affords improved postoperative outcomes and survival when compared to MV replacement [10]. Surgical intervention for degenerative MR consists of multiple techniques including leaflet repair with resection, chordal transfer, use of polytetrafluoroethylene neochordae, and prosthetic ring or band annuloplasty [11].
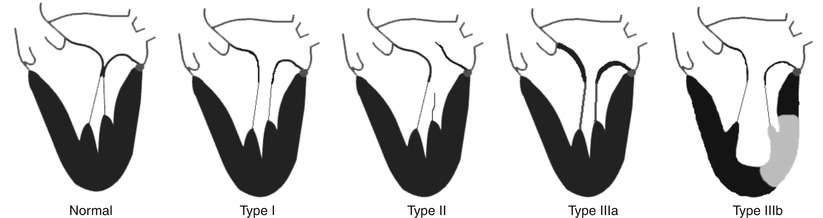

Fig. 28.2
The Carpentier classification of mitral valve regurgitation. These represent the basic mechanistic etiologies for mitral regurgitation. More than one type can coexist in a given individual. Shown at left is the normal mitral valve apparatus. Type I: normal leaflet motion with annular dilation; Type II: increased leaflet motion (leaflet prolapse or flail); Type IIIa: restricted leaflet motion in systole and diastole, usually associated with leaflet and subvalvular thickening (rheumatic); Type IIIb: restricted leaflet motion in systole, often seen in ischemic cardiomyopathy with history of infarct (light region in left ventricle) (From Feldman et al. [68], Chap. 1, p 3 with permission)
Functional MR (FMR, Carpentier Type I, or IIIb) is a direct consequence of underlying left ventricular dysfunction and annular dilation, which secondarily impairs the coaptation of otherwise structurally normal leaflets. Surgical correction of FMR has been shown to improve functional class and left ventricular remodeling [12], but a survival benefit has yet to be shown [13]. Additionally, even with current surgical annuloplasty techniques, up to one-third of treated patients have moderate or greater MR recur within 1 year of surgery [14–16]. Preoperative predictors of recurrent MR and poor outcomes after restrictive annuloplasty for FMR include LV end-diastolic diameter >65 mm [17], posterior leaflet angle ≥45° [18], and MV coaptation depth ≥11 mm [19]. It is estimated that as many as 500,000 people in North America alone have clinically significant congestive heart failure-associated MR, many of whom are currently not offered mitral valve intervention. Since even asymptomatic patients with severe MR have higher 5-year rates of death, heart failure, and atrial fibrillation, consideration of valve repair is clinically warranted in these patients. Until the advent of percutaneous mitral valve procedures, surgical mitral valve repair or replacement had been the sole option for treating MR.
Categories of Percutaneous Approaches to Mitral Regurgitation
Since mitral valve function depends on the coordinated interaction of the “mitral apparatus,” surgical therapies have been developed to address all of the individual derangements of the annulus, leaflets, chordae tendineae, and left ventricle. In cases where repair is not possible, mitral valve replacement can be performed. Percutaneous approaches have broadly mimicked surgical approaches, although several novel approaches have been developed. These technologies have been categorized as those acting on or involving the leaflets, annulus, chordal implants, LV remodeling techniques, and percutaneous mitral valve replacement (Table 28.1) [20].
Table 28.1
Percutaneous therapies for mitral valve regurgitation
Target of therapy | Device name (manufacturer) | Mechanism of action |
|---|---|---|
Leaflet repair | MitraClip (Abbott) | Clip-based edge-to-edge repair |
Percu-Pro (Cardiosolutions) | Regurgitant orifice space occupying | |
Mobius (Edwards) | Suture-based edge-to-edge repair | |
Chordal implant | NeoChord (NeoChord) | Synthetic chordae tendineae |
Indirect annuloplasty | Carillon (Cardiac Dimensions) | Coronary sinus reshaping |
Monarc (Edwards) | Coronary sinus reshaping | |
PTMA device (Viacor) | Coronary sinus reshaping | |
Mitral cerclage (NIH) | Coronary sinus—right atrial encircling | |
PS3 System (MVRx) | Transatrial coronary sinus— atrial septal shortening | |
Direct annuloplasty | Mitralign (Mitralign) | 2 × 2 plicating anchors through posterior annulus |
AccuCinch (Guided Delivery Systems) | Plicating anchors in ventricular side of mitral annulus | |
Cardioband (Valtech) | Plicating anchors on atrial side of mitral annulus | |
QuantumCor (QuantumCor) | Radio-frequency energy shrinking annular collagen | |
Millipede (Millipede) | Semirigid circumferential annular ring | |
Left ventricular reshaping | iCoapsys (Edwards) | Transventricular reshaping |
BACE (Mardil) | External basal myocardial reshaping | |
Mitral valve replacement | Endovalve-Herrmann (Endovalve) | Valve replacement |
CardiAQ (CardiAQ) | Valve replacement | |
Tiara (Neovasc) | Valve replacement |
Leaflet Repair
MitraClip Therapy
MitraClip therapy has become an important therapeutic option for patients with mitral regurgitation, and important investigations centered upon this therapy have given credence and momentum to the entire field of percutaneous mitral valve repair [21]. MitraClip therapy is based on the surgical edge-to-edge repair first described by Alfieri [22]. The surgical technique involves the use of suture to convert a regurgitant mitral orifice into a competent double-orifice valve. The inspiration for this technique came from observing the morphology of a congenital double-orifice mitral valve. The MitraClip device consists of a percutaneously delivered MRI-compatible cobalt chromium implant with two arms and two grippers used to grasp the opposing free edges of the anterior and posterior leaflet in order to improve leaflet coaptation and generate a double-orifice valve. Each MitraClip arm is 4 mm wide and 8 mm long and is covered with a polyester fabric to promote tissue ingrowth.
The MitraClip procedure is performed in a standard cardiac catheterization laboratory and requires a multidisciplinary team, including 1–2 interventional cardiologists, an echocardiographer, and an anesthesiologist. The procedure is performed under general anesthesia for patient comfort and to allow breath holding and to avoid patient movement during the delicate grasping steps. However, it is feasible to perform the procedure under conscious sedation [23]. The device is delivered from the femoral vein by a transseptal route using a steerable guide catheter (24 Fr proximally and 22 Fr at the interatrial septum), through which the steerable clip delivery system (CDS) with the MitraClip attached at the distal end is delivered. The MitraClip is steered and advanced under echocardiographic and fluoroscopic guidance into the left ventricle at the point of maximal regurgitation and withdrawn with the arms opened to allow capture of both leaflets with the grippers and arms. Once secure leaflet capture is confirmed, the MitraClip can then be tightened to improve coaptation and reduce MR further. If the desired result is achieved, the MitraClip is released from the catheter (Fig. 28.3). If the grasp is suboptimal, the clip can be reopened, the leaflets released, and the grasping procedure repeated. The procedure is performed with heparin for anticoagulation. After implantation, aspirin (325 mg) is given for 6 months and clopidogrel (75 mg) for 30 days. SBE prophylaxis is generally recommended for 6 months after the procedure. The only limitation to the number of MitraClips implanted from a safety standpoint is the development of mitral stenosis. It has been shown that MitraClip repair with one or two clips using the EVEREST inclusion criteria of a baseline MVA ≥4.0 cm2 does not result in significant mitral stenosis [24]. MitraClip therapy results in an acute post-procedural improvement in forward stroke volume and cardiac output, while decreasing LV end-diastolic pressure and end-diastolic volume [25]. MitraClip therapy is intended for the treatment of MR that has a primarily central origin (A2/P2 interface), based on the original surgical technique described by Alfieri. The MitraClip has been applied successfully to a wide spectrum of degenerative (anterior, posterior, and bileaflet prolapse) and functional pathologies with central regurgitant jets. Regardless of regurgitation etiology, the final common objective of MitraClip implantation is the generation of a double-orifice valve. Off-label use to treat lateral regurgitant jets has been described (Fig. 28.4).
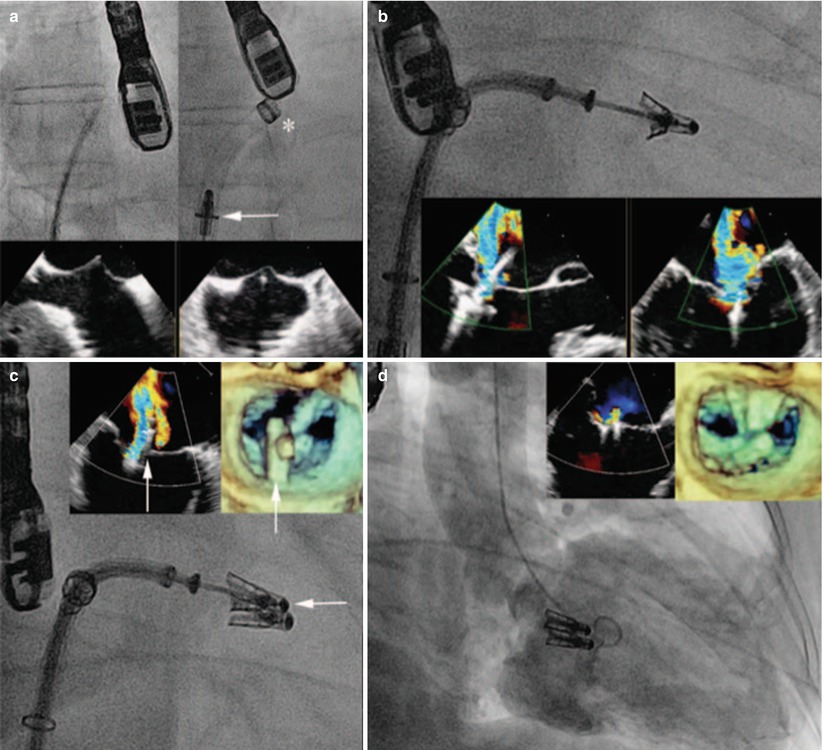
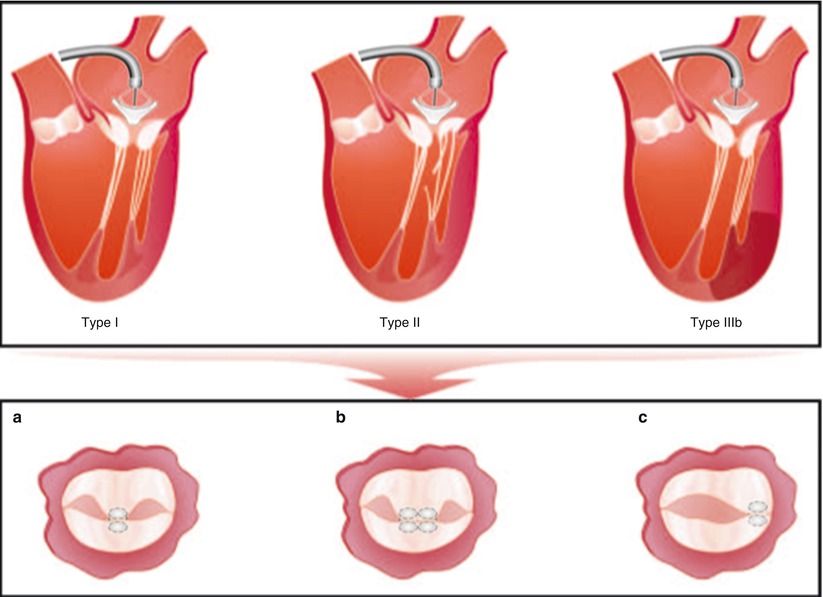

Fig. 28.3
MitraClip procedure. Although echocardiography (2-D and 3-D TEE) is the predominant modality for imaging and guiding the MitraClip procedure, fluoroscopy also provides important information. (a) Transseptal puncture is performed using standard technique under biplane TEE guidance to ensure adequate height above the mitral valve plane. The guiding catheter (asterisk) is then placed into the left atrium over a wire, through which the MitraClip (arrow) is delivered. (b) The MitraClip is positioned within the regurgitant jet using orthogonal TEE views before grasping the leaflets. (c) In this case, there was a residual regurgitant jet lateral to the first MitraClip; therefore, a second MitraClip (arrow) was positioned and deployed. (d) Two adjacent MitraClips in place after deployment with generation of a double-orifice valve with trace residual regurgitation

Fig. 28.4
Multiple pathologies are treatable with MitraClip therapy. The top panel shows multiple Carpentier-type etiologies of MR that can be treated with MitraClip therapy. Lower panel illustrates the final common pathway for MitraClip therapy, which consists of generating a double-orifice valve. One or more MitraClips can be placed centrally (a, b). For lateral or commissural jets, a MitraClip can be placed in the para-commissural region with a resultant single orifice (c) (From Rogers and Franzen [21], with permission)
EVEREST Trials
The North American EVEREST (Endovascular Valve Edge-to-Edge Repair Study) trials represent the safety and feasibility (EVEREST I) and pivotal (EVEREST II) MitraClip trials, which established the initial algorithms for clinical and echocardiographic patient selection and the procedural protocols and utilized echocardiographic core-lab follow-up.
The EVEREST I safety and feasibility trial enrolled 55 subjects and was reported with an additional 52 patients treated in the pre-randomization start-up (roll-in) phase of the EVEREST II pivotal trial [26]. Patients were selected if they were candidates for MV surgery, met major ACC/AHA indications for MV surgery, and had very specific echocardiographic criteria including a primary central regurgitant jet associated with the A2/P2 segments, a leaflet coaptation length ≥2 mm, a coaptation depth ≤11 mm, a flail gap <10 mm, and a flail width <15 mm. Key exclusion criteria included LVEF ≤25 %, LV end-systolic dimension >55 mm, mitral valve orifice area <4 cm2, or recent myocardial infarction. Of the 107 patients who underwent the MitraClip procedure in this early clinical experience, 70 (74 %) achieved acute procedural success (APS), defined as a post-procedure reduction of MR to ≤2+. Of subjects who did not achieve APS, 17 (16 %) had MitraClip implantation with acute MR grade >2+, and 11 (10 %) had no MitraClip implanted with post-procedure MR > 2+. At 12 months, not including crossover to surgery, 50 of 76 APS patients (66 %) had echocardiographic follow-up and continued with MR ≤ 2+. A total of 32 patients underwent MV surgery after a clip procedure, and when repair was planned, 84 % were successfully repaired. Freedom from death at up to 3 years follow-up was 90.1 %, and freedom from surgery was 76.3 %. The majority of patients included had degenerative MR (79 %), but patients with functional MR (21 %) had similar acute results and durability.
The landmark EVEREST II trial was the first randomized trial of a percutaneous mitral repair device measured against conventional surgical therapy and also represents the first echocardiographic core-lab adjudicated follow-up after surgical mitral valve repair [27]. This trial randomized 279 patients with 3–4+ MR amenable to surgical repair prospectively to receive either MitraClip therapy (n = 184) or conventional surgical repair or replacement (n = 95) in a 2:1 ratio. The primary safety endpoint was any major adverse event (MAE) at 30 days. Prespecified MAEs were death, major stroke, reoperation of mitral valve, urgent/emergent CV surgery, myocardial infarction, renal failure, deep wound infection, ventilation >48 h, new onset permanent atrial fibrillation, septicemia, GI complication requiring surgery, or transfusion ≥2 units. The composite primary efficacy endpoint was defined as 12-month-freedom death, new surgery for mitral valve dysfunction, or MR > 2+. The intention-to-treat 30-day MAE rate was lower in the MitraClip group when compared to the surgery group (15 % vs. 48 %, superiority P < 0.001). The MAE difference was driven largely by transfusion requirements in the surgical arm; however, in response to this criticism, a modified MAE analysis which defined major bleeding as transfusion ≥4 units or requiring surgical intervention still demonstrated a lower MAE rate in the MitraClip group (5.0 % vs. 30.9 %, P < 0.0001) [28]. Primary clinical efficacy at 12 months by intention to treat was achieved in 55 % of the MitraClip device patients and 73 % of the surgery group (non-inferiority P = 0.007). After 2-year follow-up, primary clinical efficacy was preserved in 52 % of the percutaneous-repair group and 66 % in the surgery group (P = 0.04, non-inferiority). There was no need for emergency surgery in any patient treated with MitraClip therapy. It is important to emphasize that the EVEREST II trial examined the safety and efficacy of MitraClip therapy as an initial strategy in the treatment of patients with MR. Not every MitraClip procedure was successful in reducing MR, but of these failures in reaching acute procedural success, the majority went on to have successful subsequent mitral valve surgery. Both the MitraClip and surgically treated subjects had favorable LV remodeling and improvements in quality of life at 12 months. A non-prespecified subgroup analysis showed that for subjects with age ≥70 years, functional MR, and LVEF <60 %, surgery was not superior to percutaneous treatment with regard to efficacy. Concomitant with the EVEREST II trial, a single-arm “high risk registry” (HRR) enrolled 78 patients with primarily FMR and an STS or surgeon-estimated surgical procedural mortality risk of ≥12 %. The HRR demonstrated superior safety compared to the estimated surgical risk, as well as favorable LV remodeling and improved symptoms in most patients.
It is desirable that subsequent surgical options for MV repair are preserved after MitraClip implantation. This is most important in low surgical risk patients with degenerative MR. However, it is debatable whether mitral valve repair after a failed MitraClip procedure in functional mitral regurgitation should even be attempted, as a mortality difference has not been shown between repair and replacement in patients with ischemic MR up to 6 years after surgery [29]. Nonetheless, it has been shown that surgical repair can be performed in many subjects successfully after MitraClip therapy, with successful repair having been reported up to 5 years after implantation [30, 31]. It is clear that not every patient will be able to have successful surgical repair after MitraClip therapy, and it is very likely that fibrosis over time will hinder successful clip removal without injuring the leaflets. The degree to which this limitation will be clinically significant requires further study. The presence of anterior/bileaflet flail or prolapse has been shown to be a significant predictor of the need for MV replacement. However, surgeon experience (defined as the number of surgeries performed annually), duration of implant, or an operative note of valve injury or difficulty removing the device did not have an impact on whether a repair or replacement was performed [32].
European Registry Results
In March 2008, the MitraClip received CE-mark approval. Since then, the majority of MitraClip implants have been performed in Europe. In addition, the clinical characteristics of European patients are different from those enrolled in the EVEREST trials, as the relatively limiting mitral leaflet anatomic criteria were not consistently observed. The two largest reports to date on the European MitraClip experience emanate from a single center in Germany [33] and two centers in Italy [34], for a total of 82 patients. Both centers reported favorable acute and short-term outcomes in patients at high risk for surgery with primarily FMR and low ejection fractions. Data from the EVEREST trials and European registries are summarized in Table 28.2.
Table 28.2
Comparative summary of major MitraClip clinical trials
% (Per) | EVEREST/Non EVEREST eligibility | Degenerative/Functional ecology | Age, Mean | Baseline LVEF % | STS Score/Logistic EuroSCORE, Mean | Baseline NYHA II/IV, % | Follow-up NYHA II/IV, % | Mean MR grade at baseline | MR ≤2+ at follow-up | Echocardiographic Core Lab | Procedural mortality/In hospital death | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
EVEREST and EVEREST II | 107 | 100 %/0 % | 79 %/21 % | 71 | 62 | NR/NR | 46 % | 8 % (at 12 months) | 3.3 | 74 % (discharge)/ 66 % (12 months) | Yes | 0(0 %)/1(0.9 %) |
Roll-In Registry (USA), Feldman et al. | ||||||||||||
EVEREST II | 184 | 100 %/0 % | 73 %/27 % | 67 | 60 | NR/NR | 52 % | 2 % | 3.2 | 79 % (12 months) | Yes | 0(0 %)/2 (1 %) @ 30 days |
Randomized Trail (USA), Feldman et al. | ||||||||||||
German Experience Franzen et al. | 51 | 31 %/69 % | 31 %/69 % | 73 | 36 | 16/28 | 98 % | 33 % (at discharge) | 3.6 | 94 % (at discharge) | No | 0(0 %)/ 0(0 %) |
Italian Experience Tamburino et al. | 51 | 100 %/0 % | 42 %/58 % | 71 | 42 | 10.3/14.3 | 87 % | 0 % (at 30 days) | 3.9 | 97 % (30 days) | No | 0(0 %)/NR |
In the German experience, MitraClip therapy was used in 51 patients with ≥3 + MR who were at high risk for mitral valve surgery based on a logistic EuroSCORE >20, an STS score >12, or by other clinical factors which the team felt conferred a high surgical risk. Patients in this series had worse LV function and more adverse valve morphology than those enrolled in the EVEREST trials. Specifically, 35 patients (69 %) had LV or leaflet characteristics that would have excluded them from enrollment in the EVEREST trials. Despite these adverse characteristics, acute outcomes were favorable, with reduction in MR severity two or more grades in 65 % of subjects and no clinically significant mitral stenosis, procedure-related major adverse events, or inhospital mortality. Longer-term outcomes from this cohort have not been reported, and therefore effects on prognosis and durability of left ventricular remodeling and MR reduction remain unanswered.
In two Italian centers, 31 subjects who were high risk for surgery underwent MitraClip therapy. Although clinical and anatomic leaflet criteria were similar to the EVEREST trials, there was no exclusion for very low LV ejection fraction or severe LV enlargement. Acute device success was observed in 97 % of patients, with sustained MR reduction and favorable LV remodeling seen at 30 days. The higher acute success rates in this series as compared to the EVEREST trials may reflect the lack of a blinded core lab, a different patient population, more liberal use of two clips, and an accelerated procedural learning curve as a result of prior experience in North America. Longer-term efficacy and durability in this initial cohort has not yet been reported. Hopefully the ongoing North American REALISM registry will validate these outcomes.
MitraClip Future Directions
The MitraClip has emerged as an important therapeutic option for patients with mitral regurgitation. The device has excellent safety; acute outcomes are favorable, and for those patients with acute procedural success, midterm durability (up to 2 years) is reasonable. For patients at low surgical risk with degenerative MR, surgical mitral valve repair has set a high standard, with a 10-year freedom from reoperation of up to 97 % [35]. Based on adoption patterns in Europe, recent utilization trends in North America, and subgroup analyses from the EVEREST II trial, it would appear that patients who are older, at higher risk for surgical therapy, or with functional MR and depressed ejection fractions will constitute the initial target population for MitraClip therapy. Since functional MR is primarily a ventricular problem, it remains to be seen whether a pure leaflet intervention will have durable efficacy or will be superior to best medical therapy with cardiac resynchronization therapy. If so, a very large population of patients could benefit. The potential importance of concomitant annuloplasty as an adjunct to MitraClip remains unanswered. Failure to perform annuloplasty at the time of surgical degenerative leaflet repair has been shown to adversely affect repair durability [36–38]. Future iterations of the MitraClip may simplify leaflet insertion and grasping. 3-D transesophageal echocardiography (TEE), which was not widely available at the outset of the MitraClip experience, has been shown to lessen procedure times and improve visualization [39].
Other Leaflet Repair Technologies
The Mobius device (Edwards Lifesciences, Irvine, California) uses an innovative transseptal catheter with suction capability to grasp the leaflets and deliver suture to create a double-orifice valve. The procedure was performed successfully on 15 patients in 4 international centers with acute reduction of MR by ≥1 grade in 9 patients and sustained improvement in 6 patients at 30 days. Due to the limited clinical benefit and lack of durability, human clinical development was abandoned [40]. However, if in the future the technical aspects of suture delivery can be improved, the procedure may yet have utility.
The Cardiosolutions Percu-Pro™ System (Cardiosolutions Inc., Stoughton, MA) is a percutaneously delivered polyurethane-silicone polymer “Mitra-Spacer.”
This is a balloon-shaped spacer that occupies the regurgitant mitral orifice and is anchored to the left ventricular apex (Fig. 28.5). Animal studies have shown that the device is nonthrombotic with minimal inflammatory response. Human implants are planned.
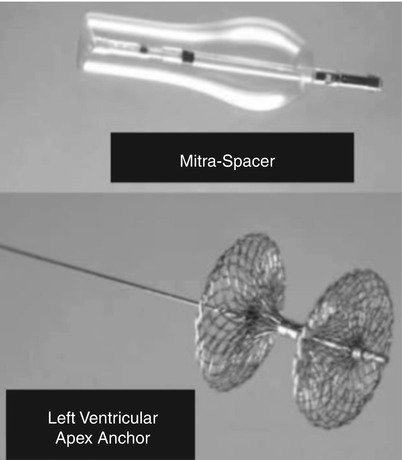

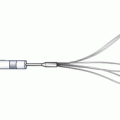
Fig. 28.5
The Percu-Pro system. The space occupying Mitra-Spacer balloon acts as a “buoy” filling the mitral regurgitant orifice and is anchored by a tether to the left ventricular apex
Chordal Implantation
NeoChord
The NeoChord device is a beating-heart, sternal-sparing, transapical approach to implantation of artificial chordae tendineae in patients with leaflet prolapse or flail (Fig. 28.6). The LV apex is exposed via a left lateral mini-thoracotomy. The procedure is guided by TEE, and a catheter is used to grasp the leaflets and attach artificial chordae that are anchored to the LV apex. The system utilizes a unique fiber-optic feedback system to ensure the target leaflet is appropriately centered in the jaws of the device. In addition to being minimally invasive, the beating-heart aspect of the procedure allows the operator to assess real-time reduction in MR by varying chord tension. The European TACT Trial (Transapical Artificial Chordae Tendineae) is underway and targets subjects with severe degenerative mitral regurgitation.
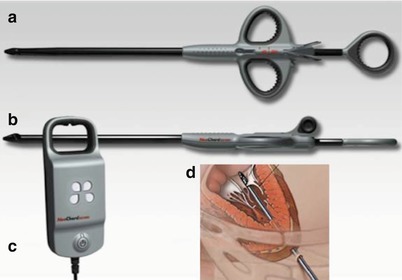

Fig. 28.6
NeoChord device. The NeoChord device is delivered from a transapical approach and consists of a catheter to grasp the leaflet (a), an exchangeable cartridge containing ePTFE suture (b), and an integrated fiber-optic display to verify leaflet capture (c). Panel (d) shows the system in use
Annular Shape Change Approaches
Surgical annuloplasty is an effective stand-alone treatment for functional MR and is an important adjunct to leaflet repair procedures for degenerative mitral regurgitation. Percutaneous techniques have been developed to mimic surgical annuloplasty. Most approaches have attempted to reshape the annulus “indirectly” by reshaping the coronary sinus. Other techniques employ more “direct” annuloplasty by attaching a series of anchors near or on the annulus which can be drawn together (plicated) with suture, with the goal of reducing the mitral annular circumference and the septal-lateral dimension. Finally, technology is under development to completely replicate a surgical annuloplasty with a semirigid implantable circumferential annular ring.
Indirect Annuloplasty
The coronary sinus (CS) travels in close proximity to the mitral annulus (Fig. 28.7




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree