Percutaneous Transhepatic Cholangiodrainage
20.1 Basic Principles
Several basic methods are available for accessing the bile ducts through an interventional, nonoperative approach:
Endoscopic retrograde cholangiography
Percutaneous transhepatic cholangiography and drainage
Endosonographically guided cholangiography and drainage (EUS-CD), which is discussed in Chapter ▶ 22
Today, diagnostic indications have become less important than therapeutic indications owing to the quality of imaging procedures such as EUS and magnetic resonance cholangiopancreaticography (MRCP). Endoscopic retrograde cholangiography (ERC) has become the procedure of first choice at most centers. Percutaneous transhepatic cholangiography is generally used if ERC fails for some reason or will very likely be unsuccessful. Endosonographically guided cholangiography and drainage (EUS-CD) has not yet become widely established and is practiced at only a few select centers.
Percutaneous transhepatic cholangiography (PTC) and percutaneous transhepatic cholangiodrainage (PTCD) are procedures for visualizing (by contrast injection) and treating (by drainage, dilatation, and other means) the extra- and intrahepatic biliary tree. The combination of endoscopic and percutaneous techniques is called the rendezvous technique. Rapid technical advances and refinements in instrumentation have constantly expanded interventional capabilities. PTC offers particular advantages over endoscopic drainage techniques in the hilar region and in the management of intrahepatic strictures.1–3
Surgery is the treatment of choice for biliary tract tumors that are amenable to primary curative treatment.
Treatment in the palliative setting (inoperable patient or high comorbidity) should be symptom-oriented. Biliary obstruction is managed by primary endoscopic treatment or alternative drainage procedures (PTCD, EUS-CD). These procedures require a shorter hospital stay than surgery. Today, even patients with benign biliary tract diseases may consistently be managed nonsurgically as a result of technical advances and growing experience with interventional techniques.
Ultrasound-guided PTC(D), with its capacity for real-time imaging in three dimensions, can provide a higher success rate and a significantly smaller number of failed attempts, especially for beginners.4–6 It has also been shown that the added use of color Doppler ultrasound can further reduce complication rates.7 Remarkably, however, ultrasound-guided percutaneous techniques have not become established at all institutions, especially those that continue to rely on radiography. Other arguments in favor of ultrasound guidance are a decrease in fluoroscopy time and less radiation exposure to patients and staff. The accidental puncture, for example, of the gallbladder or other cystic structures will certainly become less frequent, despite the lack of “hard” controlled data to support this claim.
The success of the procedure depends on the individual experience of the operator, the quality of the equipment, and patient-specific factors. A “two-person technique” is helpful during the placement of a drain.
20.2 Indications
In principle, endoscopic retrograde cholangiopancreatography (ERCP) has become the standard procedure and is superior to percutaneous transhepatic cholangiodrainage in its success rates and side effects, especially in nondilated bile ducts. PTC and PTCD are indicated in cases where a simpler and less risky8,9 endoscopic procedure is not feasible for diagnosis and treatment (e.g., inaccessibility following the creation of a biliary–enteric anastomosis, a Billroth II operation, gastrectomy, hepaticojejunostomy, a Roux-en-Y choledochojejunostomy with a failed afferent limb, duodenal diverticula, etc.), or cases in which endoscopic approaches have failed (e.g., unpassable stenosis,). If we include centers with experienced operators and access to specialized equipment (papillotomes and balloon-based forward-viewing enteroscopes), we find that the endoscopic approach is likely to be successful in <70% of patients who have postsurgical anatomical changes in the gastrointestinal tract.10 Some centers employ PTC as their primary technique for extensive hilar tumors owing to its higher success rates.
Patient selection criteria are very similar to those for endoscopic procedures (location and extent of the biliary tract obstruction and its drainage):
Cholangiolithiasis (intra- or extrahepatic)
Neoplasms
Congenital anomalies
Immune-related diseases (e.g., primary sclerosing cholangitis [PSC]) and their sequelae,11,12 also bacterial (secondary sclerosing) cholangitis
Other indications are postoperative and other posttraumatic changes such as bile leaks and complications after liver transplantation. Biliary tract imaging may also be necessary to detect or exclude a communication in patients requiring abscess drainage or the local ablative treatment of a parasitic disease (e.g., puncture–aspiration–injection of alcohol–reaspiration [PAIR], Chapter ▶ 17). Today the indication for PTC is almost always therapeutic—the decompression of an obstructed biliary tract—and drainage procedures are most frequently performed.13
20.2.1 Endoscopic Retrograde or Percutaneous Approach
If surgery is not indicated in a patient with malignant obstructive jaundice, it must be decided whether to proceed with the endoscopic retrograde or percutaneous implantation of a drain or stent. Stanley (1986) compared percutaneous and endoscopic techniques alone and combined with surgical intervention and emphasized the lower rate of bleeding complications, higher patient acceptance, and avoidance of an extracorporeal catheter system as the advantages of endoscopic retrograde treatment.14
In 1987, Speer recommended endoscopic retrograde stenting via the papilla of Vater as a primary treatment for elderly patients in poor general health. A randomized study comparing endoscopic and percutaneous stent insertion for malignant occlusive jaundice found that the endoscopic retrograde approach was associated with a significantly higher regression of bilirubinemia and a significantly lower 30-day mortality rate.15
20.2.2 Rendezvous Technique
Sommer (1987) reported on the combined use of endoscopic and percutaneous approaches in the “rendezvous” technique, also viewing the placement of an internal biliary drain as the treatment of choice for malignant common duct strictures no longer curable by surgery. Here a thin drainage is placed through the liver into the duodenum for the endoscopic access and a guidewire is placed over the papilla and grasped with an endoscopic forceps. Then a prosthesis can be placed over this wire to achieve biliary drainage. In a significant proportion of patients the endoscopic access is possible without transhepatically placed wire shortly after removing the PTC drain since the papilla has been slightly dilated and can be intubated easily. With this technique, a stent can be placed even after Billroth II surgery or with a duodenal diverticulum restricting endoscopic papillary access, and long proximal stenoses can be crossed and stented.16 In 1985, Shorvon described a successful papillotomy using the rendezvous technique in 10 of 11 cases where endoscopic technique had failed due to large duodenal diverticula or a prior Billroth II gastrectomy.17 Another advantage of the rendezvous technique, noted by Hauenstein, is the ability to place a large-bore endoprosthetic stent without painful dilatation of the percutaneous transhepatic tract.18,19 Indications for the percutaneous implantation of biliary drains are listed in ▶ Table 20.1.
| State/Intention | Situation |
| Preoperative when endoscopic retrograde approach is not possible |
|
| Curative |
|
| Palliative |
|
20.3 Contraindications
PTCD has the same contraindications as any liver puncture. Lack of informed consent, comorbidity that affects prognosis, coagulation disorders (Quick prothrombin <50%, PTT >50 seconds), and thrombopenia (<50 × 109/L) should be corrected prior to the intervention. The presence of ascites (decompensated hepatic cirrhosis with neoplasia) may necessitate preliminary fluid drainage if conservative therapies are unsuccessful (Chapter ▶ 13). Ascites is associated with an increased risk of biliary peritonitis and hemorrhage. Contrast allergy is considered a relative contraindication and, if the procedure is definitely required, calls for suitable premedication with corticosteroids and antihistamines (e.g., 150 mg prednisolone, H1 and H2 blockers) following adequate informed consent. Contraindications should be viewed as relative in septic patients and when no alternatives are available.
20.4 Materials and Equipment
20.4.1 Description of Materials
The following are required:
Fluoroscopy unit
Sterile gloves, gown, and drapes
Sterile ultrasound probe covers
Sterile table with sufficient sponges
Hypodermic needle, 22 gauge, 0.7 mm diameter, length 3 cm (Braun Melsungen)
Large supply of 5-mL, 10-mL, and 20-mL syringes, assorted syringe volumes for different fluids to avoid confusion: e.g., local anesthetic (5-mL syringes), 0.9% isotonic saline solution (10-mL syringes), and radiographic contrast medium (20-mL syringes)
Local anesthetic such as 1% prilocaine hydrochloride
Radiographic contrast medium such as Conray 30 (Mallinckrodt Medical), 50 mL
Sterile dishes for 0.9% NaCl
One three-way stopcock
Saline solution, 50 mL
Skin prep solution such as octenidene (Octenisept, Schuelke), scalpel (pointed), needle holders, scissors, forceps, dressing and sponge holding forceps
Hollow needles with stylet (e.g., Chiba needles), 20 gauge and/or 22 gauge
Wire, 60 to 90 cm, 0.018-inch, soft with very soft tip
Wire, 90 cm, 0.035-inch, stiff with soft tip
Insertion catheter, 5F or 6F, straight, mounted on blunt metal cannula
Dilators, 8F and 10F
Internal/external drainage catheter; 8F, 10F or 12F, with pigtail and side holes for long-term drainage (e.g., Biliary Plus drain, Peter Pflugbeil)
Collecting bag
Suture material, Mersilene 0 MH plus, 36 mm 1/2 c, polyester (Ethicon)
Dressing material
Puncture needles Suitable types: 22-gauge Chiba needle, diameter 0.7 mm, length 22 mm, disposable (Pajunk); 20-gauge puncture needle (1.1 mm), length 20 cm; 18-gauge puncture needle (1.3 mm), length 20 cm with inserted movable trocar (Pajunk). It should be noted that 20-gauge needles deliver a better steering and ultrasound visibility despite the 22-gauge needle being the standard version. In the standard procedure a blind puncture technique is used, demanding several needle passes. US-PTCD is frequently performed with 1 or 2 needle passes.
Guidewires The classic standard guidewires for PTCD are 0.018-inch, length 60 cm for changing from the puncture needle to the insertion set, and 0.035-inch, length 90 cm. Guidewires longer than 90 cm are more cumbersome to handle (Chapter ▶ 22). Other guidewires are available for special applications: straight, 0.018-inch, nitinol, platinum tip, length 120 cm (OptiMed); Terumo guidewire, curved, 0.035-inch, flexible 10-mm tip, length 180 cm (Terumo Europe); stiff guidewire, Bardselect Ultra-Torque GW (Black Wire), 0.035-inch, flexible 8-cm tip, length 145 cm (Bard Angiomed). The usage of uncoated wires in puncture needles is recommended to avoid shearing of the coating.
Insertion set PTC drainage set loop catheter, length 40 cm, 6F (2 mm) or 7F (2.33 mm) (Bard Angiomed); 6F or 20201 051 (7F) bile duct initial puncture set with platinum marker, catheter diameter 6F, catheter length 20 cm, guidewire 0.018-inch, length 80 cm (OptiMed) or Dilplus metal dilator with 5F Teflon catheter, length 27 cm (Peter Pflugbeil).
Dilators 6F to 10F dilators (e.g., Bard Angiomed); Nimura bile duct dilator, 8F to 16F, length 60 cm (Peter Pflugbeil).
Drainage sets 8F to18F with side holes, length 34 cm (distance from skin 7.5 cm) with connecting tube, sealing cap and skin plate (Peter Pflugbeil). Other sets for PTCD are available from various suppliers (Cook Medical, Endoflex, and many others). The sets usually include a puncture needle, two wires, a dilator, and a PTCD catheter (angled ring catheter with a pigtail end and multiple side holes). Generally the catheters are available in sizes from 7F to 10F (or 12F). Some companies also offer introducer drains, usually consisting of a 5F catheter with a straight end that can function as a sheath. We have had good results with the Pflugbeil system, in which the Teflon catheter is mounted on a blunt metal dilator allowing for uncomplicated insertion of the drain. The sheath material also allows it to remain indwelling for some time. When the drains are placed under ultrasound guidance, it is helpful to choose the best introducing cannula for that purpose. A 20-gauge cannula with an echogenic tip is ideal, while a standard 22-gauge cannula is often difficult to image with ultrasound. We prefer to assemble a customized set. Ours consists of a 20F Chiba needle plus a 60-cm-long 0.018-inch guidewire, a 90-cm-long 0.035-inch guidewire, two dilators (8F and 10F, each 20 cm long), a 10F Biliary Plus angled drainage catheter (30 cm, 24 side holes, pigtail with 9.5-cm perforated distal end), and a Dilplus Teflon catheter (27 cm, 5F) mounted on a metal dilator (Peter Pflugbeil).
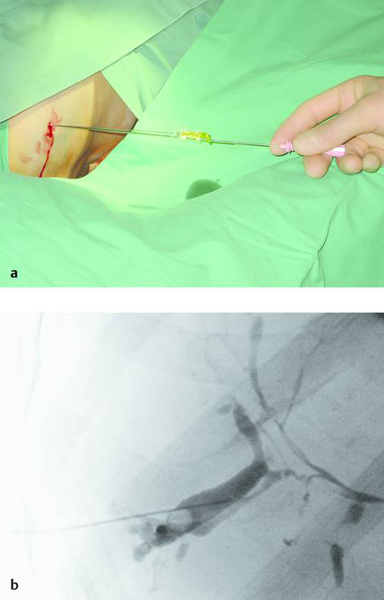
Materials necessary for percutaneous transhepatic cholangiography and drainage (PTCD) are illustrated in ▶ Fig. 20.1.
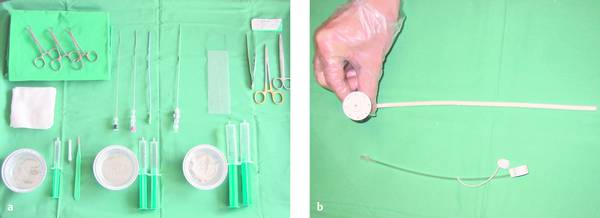
Fig. 20.1
a Materials required for percutaneous transhepatic cholangiography and drainage (PTCD).
b Munich drain (Peter Pflugbeil), size 12F.
20.5 Technique
Three basic methods of biliary tract drainage are available and can be combined with one another as needed:
Purely external percutaneous biliary drainage
Combined internal and external drainage
Placement of an endoprosthesis or stent without necessity of percutaneous tract for internal drainage of the bile ducts
A combined approach can lengthen survival time by approximately 3 months over external drainage alone, but the real breakthrough came with the introduction of large-bore endoprosthetic stents.19
Biliary stenting has the following advantages over external catheter drainage:
Greater drainage efficiency due to the larger lumen
No bile loss from the enterohepatic circulation
Better patient tolerance
No problems with drain migration or dislodgment
No need for daily flushing with NaCl
Less risk of contamination from duodenal juice in the bile ducts, no aspiration of duodenal juice
Preservation of papillary function, also with respect to the pancreatic duct
PTCD is a percutaneous procedure that includes the following basic steps. A percutaneous needle is introduced into a bile duct and contrast medium is injected. Next a sheath is inserted into the bile duct, with or without additional contrast injection (cholangiography); the papilla is crossed and permanent drainage is established (drain placement).
These steps may be completed in one intervention or in multiple stages. Analogous to surgical treatment, a multistage approach is advisable in critically ill patients. In a septic patient, for example, primary external drainage is done to decompress the bile ducts. Once the patient has improved clinically, internal drainage can be established.
The complete procedure is outlined below:
Benefits (target parameters, indications) are weighed against risks (risk factors and contraindications) taking into account possible alternatives (endoscopic treatment, including EUS-CD, and surgery). The steps in the procedure are described and informed consent is obtained.
Preinterventional imaging results are reviewed (conventional sonography, ERC attempts, MRCP), and an optimum access route is identified.
Coagulation status is assessed (see under Contraindications above); blood typing is optional according to individual patient risk.
Preinterventional antibiotic therapy is planned (to select a suitable antibiotic that is excreted in the bile, such as ceftriaxone or ciprofloxacin).
Current endocarditis guidelines are reviewed.
The patient is adequately fasted before the procedure (generally 6 hours).
Instrument and equipment setups are prepared.
Standard sedation/analgesia is prepared with propofol (e.g., initial additional bolus of midazolam, 1–2.5 mg IV), fractionated during continuous pulse oximetry, circulatory monitoring, and assessment of consciousness (level of sedation; see Chapters ▶ 11 and ▶ 22). Analgesic use is optional but is widely practiced (e.g., meperidine hydrochloride [pethidine], 50 mg, or another morphine derivative).
The patient is positioned supine on the fluoroscopy table.
The puncture site is localized with ultrasound (right intercostal, epigastric).
Local anesthesia is administered and the skin incision is made.
The needle is introduced under ultrasound guidance.
Bile is sampled (optional).
Contrast injection and fluoroscopy are carried out and documented.
A 0.018-inch guidewire is introduced.
A sheath is inserted (e.g., Dilplus, Peter Pflugbeil) to exchange for a 0.035-inch catheter, and access is secured for further manipulations (internalization).
The needle tract is dilated with Seldinger technique up to or up to 2F less than the proposed drain size.
The percutaneous biliary drain is placed and its placement is documented.
The incision is sutured and dressed.
The drainage setup is completed.
Postinterventional monitoring protocol after sedation/analgesia is implemented.
Drain care is recommended (flushed several times daily, at least twice, and output is recorded; in case of internal–external drainage, decision for complete internal drainage or external drainage).
Further interventional management is planned including internalization of drainage (or long-term drainage with drain changes at approximate 3-month intervals), metal stent insertion, lithotripsy (may include cholangioscopy), and other procedures such as photodynamic therapy or brachytherapy.
20.5.1 Patient Positioning
The patient position and equipment setup are illustrated in ▶ Fig. 20.2.
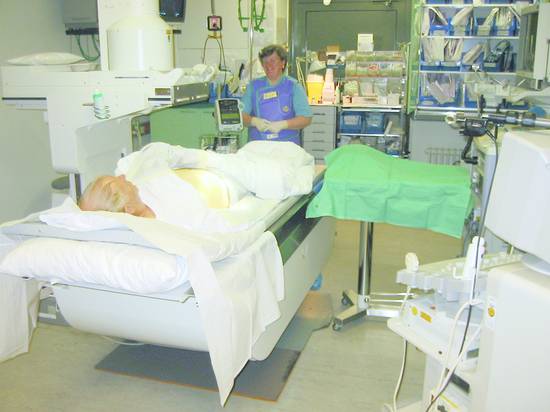
Fig. 20.2 Patient positioning and equipment setup (ultrasound scanner, fluoroscopy unit, monitoring unit, instrumentation). The instrument stand, ultrasound scanner, C-arm fluoroscopy unit, and operator stool are shown.
20.5.2 Needle Insertion and Drainage
Puncture Site and Access Route
The location of the puncture site conforms to drainage requirements (left or right lobe of the liver). The puncture site may be intercostal (10th–11th intercostal space); right anterolateral (just anterior to the midaxillary line, usually caudal to the 10th rib), for drainage of the right hepatic duct and its anterior and posterior side branches; or epigastric (subcostal) for drainage of the left hepatic duct. The angle of needle insertion (horizontal and slightly cephalad) should ensure that the needle tip will enter a peripheral bile duct at an approximately parallel (tangential) angle. Color Doppler imaging is helpful for avoiding the undesired puncture of portal vein branches.
Needle Orientation for Conventional PTCD
For an intercostal approach directed toward the porta hepatis, the needle is oriented roughly horizontal to the radiography table (and perhaps angled slightly cephalad), aiming it toward the contralateral shoulder or the transverse process of the T12 (or L1) vertebral body.20 With an epigastric approach, the needle is directed toward the porta hepatis while angled approximately 30° to 50° to the table surface.
Local Anesthesia
Local anesthetic is infiltrated down to the liver capsule (22-gauge needle with 10 mL of 1% prilocaine hydrochloride). The needle is introduced along the superior border of the lower rib to avoid injury to intercostal nerves and vessels (▶ Fig. 20.3).
Fig. 20.3 A stab incision is made following local anesthesia.
Conventional Puncture of the Biliary Tree and Cholangiography
The biliary tree can be punctured in one or two steps using a single-needle or two-needle technique. The puncture needle is advanced into a peripheral bile duct under ultrasound guidance or 4 to 6 cm deep to the liver capsule using “blind” fluoroscopic technique. Bile aspiration is attempted with a syringe. Thereafter and during further needle withdrawal, contrast medium is injected to define the biliary tree (▶ Fig. 20.4).
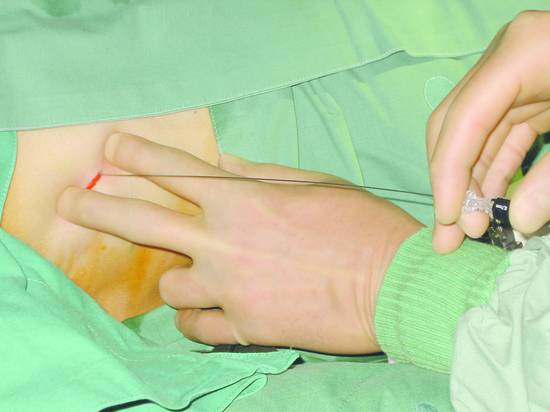
Fig. 20.4 A 0.7-mm Chiba needle is introduced blindly, without ultrasound guidance, along the superior border of the lower rib in the intercostal space.
Single-Needle Technique
An atraumatic 22-gauge (0.7-mm) Chiba needle is inserted into a peripheral bile duct in the sedated patient using freehand technique. An alternative is to use a larger-bore 18- or 20-gauge trocar system (1.1–1.3 mm; the 18-gauge needle will accommodate a 0.035-inch guidewire). As the needle is withdrawn after trocar removal, the escape of typical viscous biliary fluid will confirm correct placement. The bile is assessed for its color (turbidity), consistency, and odor, and a sample may be collected for microbiological analysis (Gram stain, culture, susceptibility testing). A small amount (0.5–2 mL) of contrast medium (e.g., Conray) can be injected to define the bile ducts (cholangiography). At this point a 5F (or 4F) sheath is introduced over a 0.018-inch guidewire to permit the insertion of a 0.035-inch wire.
Two-Needle Technique
This technique has become largely obsolete and is rarely practiced today. It involves two separate steps: primary puncture of the biliary tract with an atraumatic 22-gauge (0.7-mm) Chiba needle and contrast injection (cholangiography), followed by the insertion of a larger 18-gauge (1.3-mm) trocar system while the 22-gauge needle is still in place. The second needle is inserted parallel to the initial needle under fluoroscopic guidance.
We prefer a combination of sonographic and fluoroscopic guidance for the primary puncture of markedly distended intrahepatic bile ducts (>5 mm) with an 18-gauge (1.3-mm) needle—or with a 20-gauge (1.1-mm) needle—followed by the fluoroscopically guided intervention. An ultrasound-guided puncture requires fewer needle passes than the conventional technique, so the primary use of a thicker needle poses no difficulty. In selected cases the entire procedure can be performed under ultrasound guidance alone, using a suitable ultrasound contrast agent (0.2 mL SonoVue [or less] in 10 mL of saline solution, swirled slowly). It should be noted that the needle tip echo may be displayed slightly distal to the actual tip position. As a rule, a 20-gauge needle is easier to see with ultrasound and is easier to control than needles with a smaller diameter.
There is still no demonstration of the superiority of one technique over another in terms of success rate, complication rate, or procedure time. Rotating the fluoroscopy unit (or repositioning the patient on the X-ray table) can provide three-dimensional orientation with respect to the opacified biliary tree.
Technique of Ultrasound-Guided PTCD
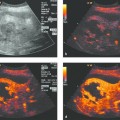
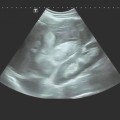
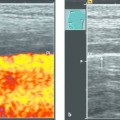
In ultrasound-guided PTCD, the initial puncture is performed under sonographic guidance (▶ Fig. 20.5, ▶ Fig. 20.6, ▶ Fig. 20.7).
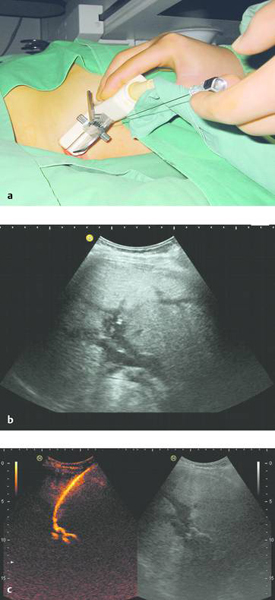
Fig. 20.5 The needle is introduced under ultrasound guidance, again at the superior border of the lower rib in the intercostal space.
a Puncture site.
b The needle is advanced to the targeted bile duct under sonographic guidance.
c Contrast administration (SonoVue) confirms correct placement in the bile duct.