CHAPTER 14
Persistent Truncus Arteriosus
Cynthia L. Rapp and Julia A. Drose
Definition
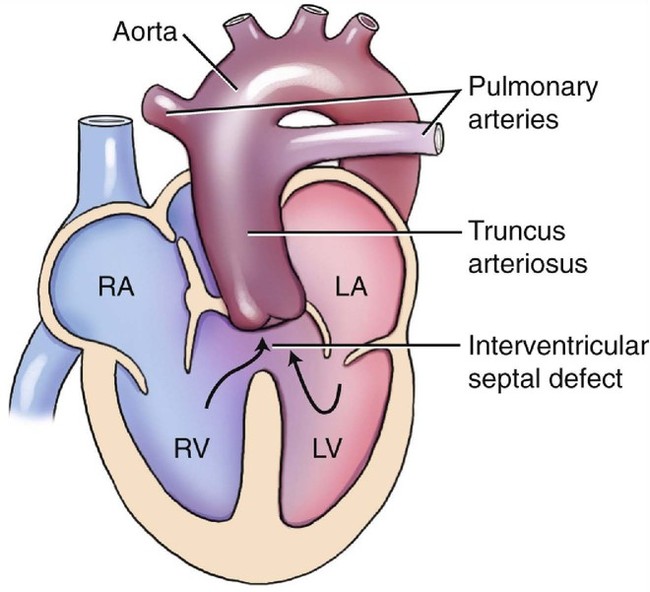
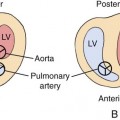
Truncus arteriosus is a rare cardiac anomaly first described in 1864.1 It is one of several truncoconal defects accounting for approximately 0.4% to 2.8% of all congenital heart defects among live births.2 In the fetus it accounts for 1% of cardiac lesions.3 Truncus arteriosus results from failure of the truncoconal ridges to fuse.4 These ridges normally divide the truncus arteriosus into separate aortic and pulmonary arterial trunks. The result is a single great vessel arising from the heart that overrides the interventricular septum. The systemic, pulmonary, and coronary circulations are all supplied with blood by this one great vessel. This failure of fusion of the truncoconal ridges also results in a ventricular septal defect (VSD). The single semilunar valve, or truncal valve, lies directly above the VSD in 42% of patients. In another 42%, it is dominantly positioned over the right ventricle, and in the remaining 16% of patients, it is positioned predominantly over the left ventricle.4 This single great vessel receives blood from both the right and left ventricles.5,6 With truncus arteriosus, the pulmonary arteries arises from the undivided truncus (Fig. 14–1).7 The single semilunar valve usually has three cusps but can have between one and six cusps.3–5,8,9 In one large multicenter study involving pathological evaluation of 536 cases of truncus arteriosus, 66% were tricuspid, 22% had four leaflets, and 11% were unicuspid. Fewer than 1% had five or six cusps.10 Truncus arteriosus is frequently associated with valve dysplasia, leading to insufficiency or stenosis. Valvular insufficiency occurs more frequently than stenosis.11 The mitral and tricuspid valves are usually normal. In approximately 50% to 75% of cases the ductus arteriosus is absent, and a right-sided aortic arch is associated 30% of the time.12,13
Truncus arteriosus has been variously referred to as aorticopulmonary trunk, truncus arteriosus communis, and persistent truncus arteriosus.1 Generally, it is abbreviated truncus arteriosus for simplicity and clarity. However, the truncus arteriosus is actually a normal embryological heart structure that becomes anomalous only when it persists throughout cardiac development; therefore persistent truncus arteriosus would be the most appropriate term to describe this abnormality.
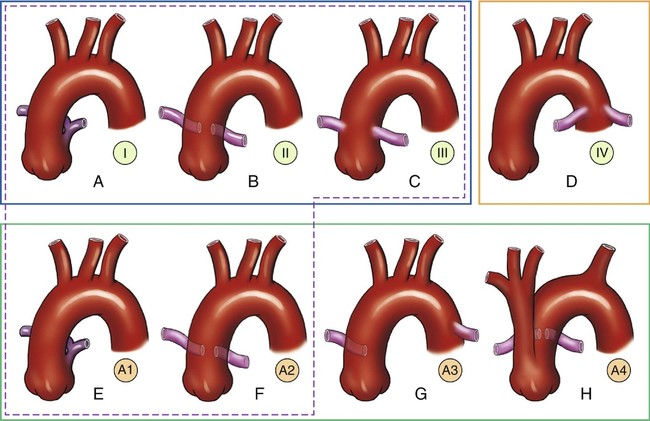
Collett and Edwards5 described an anatomical classification for persistent truncus arteriosus on the basis of the location of the pulmonary artery origins from the truncus:
• Type I: a short main pulmonary artery arising from the left lateral aspect of the truncus, then dividing into right and left branches
• Type II: separate but closely positioned right and left pulmonary arteries arising from the posterior-lateral aspect of the truncus
• Type III: widely separated pulmonary arteries arising laterally from the truncus
• Type IV: pulmonary arteries arising from the descending aorta
These divisions represent different stages of failure in the embryological development of the heart.
In 1965, Van Praagh and Van Praagh8 modified these classifications (Fig. 14–2). They proposed the following classifications:
• Type 1A: similar to the type I described by Collett and Edwards
• Type 2A: combination of type II and type III
• Type A3: a single pulmonary artery originating from the truncus, usually the right, with either a ductus arteriosus or collateral vessels supplying the contralateral side
• Type A4: truncus arteriosus with an interrupted aortic arch
The type IV classification originally described by Collett and Edwards was eliminated because it was thought to more accurately describe pulmonary atresia with a VSD. Types A1 and A2 are the most common forms of persistent truncus arteriosus, with occurrence rates of 49% and 43%, respectively. Type A3 is found in only 6% of cases and type A4 is found in 2%.8
In the fetus, the hemodynamic concern is the presence or absence of truncal valve insufficiency. Both ventricles in the fetus function as a single chamber; mixing of blood occurs by way of the VSD. If the truncal valve is competent, no significant problems are evident in utero. However, an incompetent or insufficient truncal valve can result in regurgitation of blood back into the ventricles and subsequent congestive heart failure (CHF). Soon after birth, the foramen ovale and ductus arteriosus (if present) close, resulting in a large left-to-right shunt that increases as the pulmonary vascular resistance falls. This, along with the truncal valve regurgitation that is present in about 50% of patients, results in a pressure overload to the already volume-overloaded ventricles.12,14,15 Pulmonary vascular obstructive disease usually develops by 6 months of age. Cyanosis and progressive CHF are common.16
Embryology
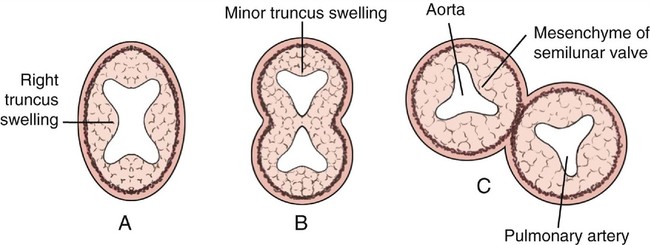
Persistent truncus arteriosus results from a failure of septation of the embryonic truncus arteriosus and the conal septum.17,18 In the normally developing heart, a single heart tube elongates and divides into ventricular and atrial components, with a small, narrow mid portion becoming the bulbus cordis.17,18 The mid portion of the bulbus cordis forms the outflow tracts of the right and left ventricles and is referred to as the cornus cordis. The inferior portion of the bulbus cordis gives rise to a short arterial trunk, the truncus arteriosus.17,18 Beginning at approximately 27 days’ gestation, spiral ridges of endocardial tissue form within the lumen of the truncus arteriosus. These ridges fuse together, resulting in the aorticopulmonary or spiral septum. It is this septum that divides the truncus into an anterior pulmonary artery and a posterior aorta (Fig. 14–3).18 Concurrently, the conal septum forms to divide the bulbus cordis. The spiral and conal septa merge, resulting in right ventricular-to-pulmonary artery continuity.18 The aortic and pulmonary valves form at the level of fusion of these septa.
Failure of the spiral septum to form results in a single great vessel and a single outflow tract. This developmental failure also produces a large defect in the infundibular septum. The size and position of the concurrent VSD is determined by the degree of deficiency of the conal septum.19 Failure of fusion usually occurs in the sixth or seventh week of gestation. This developmental failure also results in a variety of malformations of the truncal valve leaflets because two semilunar valves are normally formed from embryological tubercles on the truncal wall.17,18 The truncal valve may have thickened or poorly formed cusps. The cusps may prolapse, resulting in regurgitation. The truncal valve is anatomically continuous with the mitral valve but frequently not with the tricuspid valve.
The truncal root may be dilated as a result of receiving both the right and left ventricular components of the circulation. The ductus arteriosus may be absent, or if present it can be large in association with a hypoplastic or interrupted aorta. One of the pulmonary arteries may be absent, most commonly ipsilateral to the aortic arch. Also, the coronary arteries may vary in their origins. Failure of septation of the truncus arteriosus is thought to result from failure of neural crest cell migration.20 The fact that maternal diabetes is associated with an increased risk of persistent truncus arteriosus supports this theory.21
Occurrence Rate
Persistent truncus arteriosus is a rare anomaly, accounting for 0.4% to 2.8% of all congenital heart defects.2,5,14,22,23
As is true of most congenital heart defects, the occurrence risk in siblings of an affected infant is slightly higher than in the general population. With truncus arteriosus, the occurrence risk in a sibling is approximately 1.2%.24
In general, males have a higher incidence of cardiac anomalies and a higher incidence of lethal anomalies. However, truncus arteriosus appears to occur equally in males and females.25
Sonographic Criteria
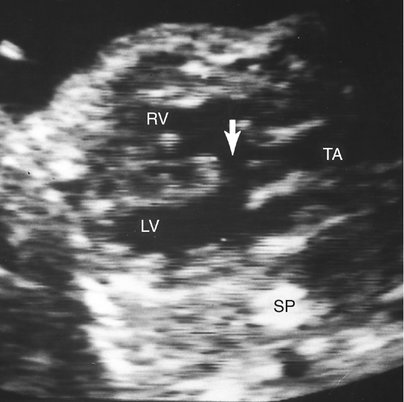
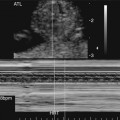
The in utero diagnosis of persistent truncus arteriosus can be challenging.26–28 However, increased equipment resolution and improved operator expertise have contributed to improved prenatal identification. The predominant finding is a single large truncal vessel overriding a VSD (Fig. 14–4). When this is present, the differential diagnosis includes tetralogy of Fallot or pulmonary atresia with a VSD. The definitive diagnosis can be made only if more than three leaflets are identified within the truncal valve or if the origin of the pulmonary arteries can be identified arising from the trunk.25
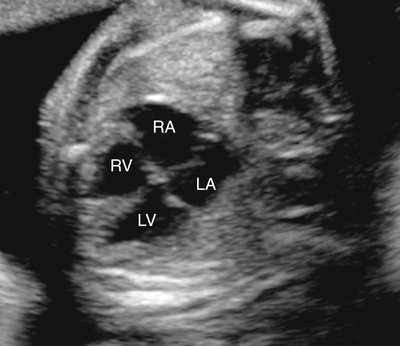
The apical four-chamber view of the heart usually appears normal in fetuses with truncus arteriosus (Fig. 14–5). Anterior angulation of the transducer from the apical four-chamber view will result in a five-chamber view, which should allow visualization of the single trunk overriding the accompanying VSD (Fig. 14–6).