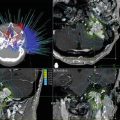
Fig. 56.1
Axial T1-weighted contrast-enhanced and T2-weighted MRI scans 4 months following GK thalamotomy
In order to discuss the merits of radiosurgical thalamotomy, it is beneficial to consider the role of conventional radiofrequency thalamotomy in the treatment of patients with tremor. Tasker retrospectively studied patients who had radiofrequency thalamotomy and thalamic stimulation [1]. He noted tremor recurrence in 15 % of thalamotomy patients, as opposed to 5 % of DBS patients. Importantly, 23 % of the thalamotomy patients required repeat procedures for tremor control. In addition, thalamotomy resulted in a decline in writing performance in 23 % of patients, as opposed to no patients in the DBS group. All of the DBS patients achieved better than 50 % improvement in dexterity, writing, and drinking ability, where only half of the thalamotomy cohort obtained that level of benefit. With respect to complications, Tasker demonstrated that chronic stimulation had a lower incidence of ataxia and dysarthria, which are common complications associated with bilateral thalamotomy [1]. Moreover, when these complications occurred in the DBS group, simply adjusting the stimulation parameters served to abolish these problems. In another study that was a prospective, blinded study, Schuurman et al. randomized 68 patients (45 with PD, 13 with ET, and 10 with multiple sclerosis) to radiofrequency thalamotomy versus VIM DBS [35]. Both groups of patients had excellent suppression of tremor, but the VIM DBS group had greater improvements in activities of daily living (ADL), and more importantly, the VIM DBS group had fewer complications. Patients who underwent thalamotomy were more likely to develop somnolence, cognitive deterioration, and dysarthria, as well as gait or balance disturbance. Thus, Tasker and Schuurman et al. have both confirmed the higher complication rates associated with thalamotomy compared with VIM DBS, and it is in this context that the use of radiosurgical thalamotomy must be considered.
As opposed to both radiofrequency thalamotomy and DBS, GKT offers a noninvasive lesioning method that does not require a skin incision, burr hole, or passage of electrodes. In addition, it does not harbor any foreign hardware placement, and the risk of infection/erosion is eliminated. Hence, the surgical risk of complications is potentially lower than that of radiofrequency thalamotomy or DBS. This technique may be ideal for patients who are not suitable to undergo a surgical procedure due to medical comorbidities or to those who want to avoid open surgical procedures with hardware implantations. GKT is effective and reported to treat parkinsonian tremor, essential tremor, multiple sclerosis-related tremor, and posttraumatic-related tremor with different results [36]. A major disadvantage of GKT is the inability to confirm the target physiologically by using MER and macrostimulation (electrophysiological monitoring). One has to rely purely on imaging-defined anatomic targeting, which lacks the accuracy of brain mapping. In addition, the method of lesion generation is delayed, and as has been suggested in some recent publications, the lesion can be variable in size [37]. Hence, GKT has both advantages and disadvantages that may give it a role in the treatment of tremor.
Compared with the large multicenter studies of deep brain stimulation for tremor, there are only a few published case series of patients treated by Gamma Knife radiosurgical thalamotomy for the treatment of parkinsonian tremor and essential tremor [6] (Table 56.1). Ohye was one of the first to advocate radiosurgical lesioning of the thalamus in patients who had a contralateral radiofrequency lesion or in patients who had a previously mapped lesion [38]. At present, Ohye has reported radiosurgical thalamotomy in 70 patients with PD and has reported tremor suppression of at least two thirds in more than 80 % of patients with no loss of effect even after 10 years in some patients [37, 39]. Duma et al. has reported their series of 42 radiosurgical thalamotomies in 38 patients over a 7-year period. In their series of patients, 24 % had complete relief of tremor, 55 % had “successful” results in improvement of their tremor, and 21 % of patients had only “mild” or no relief of tremor. Median follow-up in this series was 28 months [40, 41]. Niranjan et al. reported favorable results in 12 patients who were not candidates for open surgical procedures (nine patients with essential tremor and three patients with MS-related tremor) [42]. Eight of their 12 patients with essential tremor had complete tremor arrest, while the remaining four patients had improvement in tremor. The largest series of patients undergoing GKT has been published by Young et al. who retrospectively reviewed 158 patients who underwent radiosurgical thalamotomy [43]. Of the 74 patients with parkinsonian tremor followed at least 4 years, 79.7 % were still tremor-free, while 70.6 % of the 16 patients with essential tremor were tremor-free. In this series, only one patient sustained a transient complication, and two patients sustained permanent complications including hemiparesis and mild facial paresthesias. No cognitive changes were reported. Kondziolka et al. [44] reported a series of 31 essential tremor patients treated with SRS followed for a mean of 36 months. Of their patient cohort, 18 (66.6 %) reported improvement in action tremor and writing, 6 (22.2 %) showed improvement in action tremor alone, and 3 (11.1 %) showed no improvement. In this study, there were two complications (7.4 %) that were both permanent, including hemiparesis, dysphagia, and speech impairment. Recently, Lim et al. reported their experience with GKT for treating disabling tremor [36]. Although this study is derived from a single center, it is the only prospective blinded independent observer study that was done for GKT. In this series, assessment of tremor, ADL, complications, and long-term outcomes were analyzed in 14 patients with mean follow-up period of 7.3 months. They concluded that an improvement is achieved only in tremor and ADL, but this improvement seems to be modest, non-sustained, and less impressive than achieved with DBS. In this study, three patients developed delayed complications including hemiparesis and speech deficit.
Table 56.1
Summary of Gamma Knife thalamotomy for movement disorders
Author | Year published | Treated disorder | Target | Number of procedures | Follow-up (months) | Complication rate (%) |
|---|---|---|---|---|---|---|
Young | 1998 | PD | ViM | 27 | 22.3 (mean) | 0 |
Friedman | 1999 | PD tremor | ViM | 15 | 13 | 46.7 |
ET | ||||||
Niranjan | 1999 | 12 | 24 (median) | 8.3 | ||
Duma | 1998 | PD | ViM | 38 | 28 (median) | 0 |
Niranjan | 2000 | ET | ViM | 11 | 6 (median) | 9.1 |
MS tremor | ||||||
Young | 2000 | PD tremor | 158 | 12–96 | 1.3 | |
ET | ||||||
Mathieu | 2007 | MS tremor | ViM | 6 | 25.5 (mean) | 16.6 |
Kondziolka | 2008 | ET | ViM | 31 | 36 (median) | 6.4 |
Lim | 2010 | PD tremor | 18 | 19.2 (mean) | 16.7 | |
ET | ||||||
Young | 2010 | Essential tremor | ViM | 161 | 44 (mean) | 6.9 |
Elaimy | 2010 | ET | ViM | 1 | 144 | 0 |
Ohye | 2012 | PD tremor | ViM | 72 | 24 | N/A |
ET | ||||||
Yen | 2012 | Dystonia | ViM | 1 | 72 | 0 |
In contrast to the major studies that were done to evaluate DBS, GKT reports lack the stringent standards to prove efficacy and safety (multicenter, double-blinded, randomization, long-term follow-up). This makes it difficult to compare these different modalities; however, the results of these studies suggest that radiosurgical thalamotomy is less superior to DBS or even radiofrequency thalamotomy.
In opposition to the immediate response and relief of tremor following DBS or thalamic radiofrequency ablation, Gamma Knife radiosurgical thalamotomy effect is delayed, and different studies reported different time frames from anywhere between immediate affects to 12 months [37, 40, 42]. Niranjan et al. [44] reported immediate relief in tremor following GKT. Moro et al. [36] reported in their study of 18 patients an improvement in tremor with a delay of 6–12 months following GKT. Ohye et al. [36] reported in their study improvement in tremor starting 1 year following GKT. This gap between treatment and functional progress must be taken under consideration when making a treatment decision, since most patients with movement disorders suffer daily and some cannot tolerate the time between treatment and clinical effect. This delay is related to neurobiological sequences that take place in the brain parenchyma following GKT and the time it takes the ionizing radiation to functionally damage or to destroy the tissue targeted. The time to lesion effect can be modified and accelerated with increased doses [45]; however, the optimal technique for Gamma Knife radiosurgical lesioning has not been established. Due to the different response of tissue to ionizing radiation, lesion size can be smaller than expected, while in other cases can be larger and expand to eloquent adjacent structures resulting in serious adverse effects [46]. Peak central doses can range from 120 to 200 Gy. Duma et al. reported a difference in clinical outcome with different doses, suggesting an improvement with higher doses [41]. Despite this correlation, most surgeons at this time now perform radiosurgical lesioning for functional disorders at lower doses (e.g., 140 Gy) [42]. This decision is partially based on the series of eight patients all treated at the same institution who developed complications published by Okun et al. [46]. In that series, all except one of the thalamotomy procedures were performed at exceptionally high doses using 200 Gy (one patient was treated at 150 Gy). Unfortunately, a systematic study of dose–response complication rate has not been performed to date.
Because of the delay in effect after GKT, an accurate documentation of complications requires vigilant follow-up. The complications that can occur mainly include radiation necrosis, brain edema, and bleeding. There isn’t an established time frame for the occurrence of complication, and they can happen even years following GKT treatment. Rothstein et al. [47] reported an intrathalamic hemorrhage in the precise site of prior GKT 7.5 years after treatment. In Young’s series, 3 of 74 patients developed a complication [43]. Other reports are single-center reports of complications without a denominator to define the total number of procedures or patients who underwent GKT. Siderowf et al. reported one case of a complex, involuntary movement after Gamma Knife radiosurgical thalamotomy [2]. Unfortunately, specific dose parameters were not specified in that case report. Okun et al. reported a series of eight patients who developed multiple complications, including hemiplegia, homonymous visual field deficits, hand weakness, dysarthria, hypophonia, aphasia, arm and face numbness, pseudobulbar laughter, and death [46]. Mathieu et al. reported progressive contralateral hemiplegia following GKT with T2 changes around targeted lesion [48]. These complications all developed after treatment at different Gamma Knife centers, and it does not appear that the same rate of complications have developed at all radiosurgical centers. Nevertheless, the true incidence of complications after radiosurgical thalamotomy is not well known.
One particular explanation of the complications associated with radiosurgical thalamotomy may be related to the variability in lesion size. Animal studies in rodents and nonhuman primates have demonstrated the ability to create necrotic lesions in animal brain parenchyma [45, 49, 50]. Young et al. reported a clear correlation between lesion volume and complication. In their study, the mean lesion volume in which no complications were noticed was 188 m, while the mean volume for complications was 871 mm [51]. Dose, volume, and time are the three key factors that determine the nature of the functional ablation [45]. Higher doses can create necrosis faster as can the use of a larger-volume collimator, although almost all movement disorder GK lesions employ a 4-mm-diameter collimator. This dose–volume–time response curve can roughly be correlated in humans; however, despite this apparent consistency in dose delivery and tissue response, Friehs et al. report variable size on follow-up imaging after GK radiosurgical ablation for functional procedures. Friehs et al. examined follow-up imaging after 140 Gamma Knife radiosurgical lesions produced in the treatment of patients with PD, pain, and ET [52]. In all cases, the 4-mm collimator was used for the Gamma Knife, but the postoperative scans demonstrated lesion sizes that ranged from undetectable to 4,000 mm3. Friehs correlated the higher doses with greater lesion volume and suggested that doses of 160 Gy and above were associated with inordinately large volumes. Franzini et al. reported in their study that older patients have a higher chance of having an unexpected response, and lesion size cannot be predicted [53]. Thus, although lesion size appears to be correlated with radiation dose, there appears to be a patient to patient variability in response to Gamma Knife radiosurgical lesioning. In addition, Ohye reports two different types of lesions after radiosurgical lesions using the single 4-mm collimator isocenter with central doses of 130 Gy: “circumscribed round high signal area of approximately 7- to 8-mm diameter surrounding a smaller low signal area. The other is characterized by an irregular-shaped high signal zone extending into the medial thalamic area and/or internal capsule and often accompanied by streaking along the thalamo-capsular border” [37, 41]. Ohye speculates that the delivery time may influence lesion size. Since the cobalt sources of the Gamma Knife need to be replaced over time, delivery of the same 143-Gy dose may require double the length of time with old sources compared with new sources [37]. “After reloading, the restricted lesion was more frequent and the lesion volume was smaller.”
In conclusion, both DBS of the VIM or GKT have been employed in the treatment of tremor. Several well-conducted trials have demonstrated the efficacy and complication rate of DBS of the VIM thalamic nucleus in the treatment of both parkinsonian tremor and essential tremor. In contrast, large, well-conducted trials of Gamma Knife radiosurgical thalamotomy have not been performed. Thus, it is difficult to compare directly the efficacy and risks of GKT of the VIM nucleus for the treatment of tremor. Nevertheless, lesioning of the thalamus is an alternative treatment for tremor, and Gamma Knife ablation may be effective when properly performed. Hence, the first-line surgical therapy in the treatment of patients with medically refractory tremor is VIM DBS, and GKT remains an option for patients who cannot undergo an open surgical procedure secondary to medical comorbidities. Gamma Knife radiosurgery of the VIM thalamus, however, should only be performed by neurosurgeons who have experience in both deep brain stimulation and radiofrequency thalamic procedures to optimize results.
Pallidal DBS Versus Radiosurgical Pallidotomy
Pallidal DBS
Since its reintroduction by Laitinen and colleagues in 1992, many successful pallidotomies have been performed throughout the world in the treatment of Parkinson’s disease [54]. The most consistent effect of pallidal ablation appears to be the relief of contralateral l-dopa-induced dyskinesias, whereas the amount of improvement in contralateral bradykinesia, rigidity, and tremor differs among the many studies [55]. In contrast with the number of studies documenting the effects of ablation of the posteroventral GPi, there are remarkably few studies that extend the initial observation of Siegfried and Lippitz that chronic high-frequency stimulation of the internal pallidum may effectively treat PD [56]. GPi DBS is also becoming increasingly common procedure as STN DBS for the treatment of Parkinson’s disease. GPI DBS has also been used for the treatment of psychiatric disorders such as Tourette’s syndrome. Cannon et al. reported the implantation of bilateral GPI DBS in 11 patients with medically intractable Tourette’s syndrome. In their study, there was 48 % improvement in motor tics and 56.5 % reduction in phonic tics [57]. Other studies have showed that a unilateral GPI DBS may also be effective for the treatment of Tourette’s syndrome [58].
DBS of the STN is the most common application for Parkinson’s disease. DBS of the internal segment of the globus pallidus is also effective [54, 55]. The most consistent effect produced by pallidal DBS is a marked reduction of contralateral l-dopa-induced dyskinesias [56, 59–68]. Volkmann et al. demonstrated a 54 % improvement in the “off”-period UPDRS motor score at 1-year follow-up, with significant improvement in bradykinesia, tremor, posture, and gait. “On”-period motor symptoms did not improve significantly after surgery except for dyskinesias, which were reduced by 83 % at 1-year follow-up [68]. It appears that GPi DBS may directly reduce levodopa-induced dyskinesias, whereas STN DBS reduces levodopa-induced dyskinesias indirectly via reduction of overall medication intake. Loher et al. reported 1-year results of 16 patients showing a 38 % improvement in medication-off UPDRS motor scores and a 33 % improvement in the ADL score in patients receiving unilateral stimulation [69]. Bilateral stimulation led to a slight improvement in these results. Some improvement in the medication-on state was also noted. A larger series of 36 patients [25] demonstrated that bilateral pallidal stimulation results in a median motor improvement of 37 % and an increase from 28 to 64 % of the day without disabling involuntary movements [25]. Many reports demonstrate the beneficial effects of GPi stimulation on dyskinesias, on–off fluctuations, and tremor [3, 61, 66, 68, 70]. Also, pallidal stimulation has been validated in those patients who have previously undergone contralateral pallidotomy. In a cohort of four patients who underwent GPi stimulation contralateral to a prior pallidotomy, motor scores improved by almost 50 %, while bradykinesia was decreased by 37 % and tremor by 93 % without serious adverse cognitive or motor effects [71]. Despite the benefits of GPi DBS, most centers currently favor STN DBS in the treatment of Parkinson’s disease.
Dystonia is one movement disorder whose primary surgical target is the GPi as opposed to the STN or VIM; however, the treatment of dystonia with DBS remains in its infancy. Unlike the rapid improvement seen in PD, GPi DBS in dystonia results only in gradual improvement over several months. Preliminary results suggest that more mobile, fluid abnormal posturing may respond quicker, whereas fixed tonic abnormal posturing may require a longer period to improve [71–74]. Given these encouraging results, there have been more patients with both primary and secondary dystonia who have undergone bilateral GPi DBS. Patients with DYT1 and primary dystonia have proven to respond more favorably to GPi DBS than some of the secondary dystonias. This may relate to variability in the etiology of the secondary dystonias. Currently, DBS for dystonia is not FDA approved and has a humanitarian exemption that limits the ability for high-quality studies to be performed.
The complication rate of GPi DBS is similar to that of VIM DBS. The risk of hemorrhage, infection, and hardware-related complications are similar. Although there are no direct studies that compare the incidence of cognitive complications after bilateral pallidal DBS, the overall reported complication rate after bilateral DBS does not appear to be as high as the rate after bilateral pallidal destructive lesions. Global scores of cognitive function exhibit little change after unilateral or bilateral pallidal stimulation, although subtle worsening of frontal lobe scores including verbal fluency have been described [61, 65, 74, 75]. Risk factors for cognitive deterioration include preoperative l-dopa dosages and advanced age [74].
Despite the lack of direct comparison between the efficacy of STN DBS versus GPi DBS, most neurologists/neurosurgeons have selective preference for STN versus GPi DBS. Recently, the European NSTAPS study assessed whether GPi DBS gives grater functional improvement than STN DBS in advanced PD. This was a multicenter randomized study with a total number of 128 patients [76]. The results of this study showed improvement in the off-medication state in the STN group compared to the GPi group in the UPDRS score (20.3 versus 11.4), but failed to show any other benefit of STN DBS over GPi DBS. There are some who believe that STN DBS may have additional risk for cognitive compromise in patients who are exhibiting significant cognitive deficits. On the other hand, recent randomized, controlled multicenter studies showed that DBS of the STN does not reduce overall cognition or affectivity [77, 78]. Nevertheless, due to the potential risk for cognitive deficits following STN DBS, baseline neuropsychological studies and evaluation are important and should be done prior to surgery. Overall both STN and GPi have similar efficacies for PD; however, STN is the more common target for PD DBS, while the primary target of choice for dystonia is the GPi.
Radiosurgical Pallidotomy
The pallidotomy is a time-honored surgical procedure that was performed in the 1950s by many surgeons, including Guiot, Spiegel and Wycis, Talairach, Riechert, and Leksell. The reintroduction of Leksell’s posteroventral pallidotomy by Laitinen in 1992 (after its disappearance, which coincided with the benefits of l-dopa therapy) initiated a resurgence of interest in the surgical treatment of movement disorders. One of the most consistent benefits of radiofrequency pallidotomy is improvement in contralateral drug-induced dyskinesias. Nearly every study has documented improvement in l-dopa dyskinesias from 61 to 82 % [55, 79–87]. Tremor appears to respond by 33 to 90 % at 6 months, although tremor alone is not significant indication for pallidotomy. The results for the symptoms of parkinsonian rigidity and bradykinesia are not as consistent with studies demonstrating some benefit. In addition, there have only been minimal benefits in gait. Given the results described above, conventional pallidotomy is only recommended for patients who are severely disabled by asymmetrical l-dopa dyskinesias and who are not candidates for implanted hardware systems. Bilateral pallidotomies are not considered a good option for most patients given the high rates of cognitive complications after bilateral destructive lesions in the globus pallidus [61]. Gamma Knife radiosurgical pallidotomy differs from conventional radiofrequency pallidotomy primarily in its less invasive nature. It can theoretically expect to provide excellent relief of levodopa-induced dyskinesias and tremor with fewer complications than conventional pallidotomies.
The evidence supporting the use of Gamma Knife pallidotomy is even more sparse than the evidence supporting the use of GKT. Only case reports and case series are available for study. Indeed, the last published case series of patients treated with Gamma Knife pallidotomy dates back to 1998 [87], which most likely reflects the established success of STN DBS in the treatment of Parkinson’s disease and the high complication rates of GK pallidotomy. Friedman et al. reported four patients in 1995 who were treated with 180 Gy to the right posteroventral pallidum for advanced PD [88]. One patient had improvement in dyskinesias but also became transiently psychotic and demented. The three other patients neither improved nor suffered a complication. In a later publication, Friedman et al. reported two patients who underwent radiosurgical pallidotomy with lower doses of 120 to 140 Gy [89]. They chose to perform the procedure only in patients who had undergone a prior radiofrequency pallidotomy with physiologic mapping. They created a mirror-image lesion, assuming symmetry between the two sides. Both of these patients, however, developed complications: hemiparesis in one patient and hypophonia and swallowing difficulties in the other patient. In addition, Friedman et al. reported a stroke in one patient as a complication from GK pallidotomy [90]. This was attributed to vascular hyalinization and thrombosis. The largest series of GK pallidotomies comes from Young et al. who reported a series of 29 patients who underwent Gamma Knife pallidotomy [87]. He reports that two thirds of his patients demonstrated improvements in bradykinesia and rigidity. Only one of these 29 patients developed a complication—hemianopsia 9 months after the procedure. UPDRS scores were not consistently evaluated by blinded observers in this study. With respect to complications, Okun et al. reported three complications after Gamma Knife pallidotomy performed in one center [46]. Three patients who had Gamma Knife pallidotomy had been treated with doses of 200, 150, and 100 Gy. All three experienced visual field loss and/or hemiparesis and dysarthria.
Compared with GKT, there appears to be a shortage of published case series of patients treated with GK pallidotomy for the treatment of Parkinson’s disease. This most likely represents a lack of enthusiasm on the part of the treating surgeons and referring neurologists. There are few studies that truly document the benefit of GK pallidotomy in a blinded and unbiased fashion, and there are several reports of complications after GK pallidotomy. Hence, at this stage, GK pallidotomy is not performed as a viable method of lesioning in the treatment of PD. GK pallidotomy have been reported in case studies as a successful treatment option for dystonia and hemidystonia [91].
Subthalamic DBS Versus Radiosurgical Subthalamotomy
Subthalamic DBS
At the present time, DBS of the subthalamic nucleus is the most widely practiced surgical treatment for patients with idiopathic Parkinson’s disease (Figs. 56.2, 56.3, and 56.4). Benabid et al. were the first group to demonstrate the efficacy of STN DBS in the treatment of PD in the mid-1990s [92]. One-year follow-up of 24 patients implanted with bilateral STN DBS demonstrated that UPDRS ADL and motor scores improved by 60 % in the off-medication state [93]. The UPDRS subscores for akinesia (56 %), tremor (80 %), rigidity (68 %), and gait (55 %) also improved, in contrast with the results seen with VIM stimulation [11]. However, the effect in the on-medication state was not as pronounced, with only a 10 % improvement in the motor score noted. Levodopa drug use was cut in half. These results have held up at 5-year follow-up [94]. Kumar et al. performed a prospective, randomized, double-blinded study comparing STN DBS with the stimulator on and off [94]. They found a 65 % reduction in off-period motor UPDRS scores, a 40 % reduction in on-period motor UPDRS scores, and an 85 % reduction in l-dopa-induced dyskinesias in the seven patients who were evaluated. Limousin et al. found a 60 % improvement in off-period UPDRS scores but only a 10 % improvement in on-period UPDRS scores.




Fig. 56.2
T1 contrast-enhanced MRI scan with a superimposed reformatted Schaltenbrand and Wahren atlas with bilateral electrodes trajectory targeting the STN

Fig. 56.3
Postoperative anteroposterior and lateral skull X-rays demonstrating the implanted STN DBS electrodes

Fig. 56.4
Postoperative axial and sagittal T1-weighted MRI scans showing the implanted STN DBS electrodes
These two prospective double blind studies comparing STN DBS with the stimulator on and off found a 60–65 % reduction in off-period motor UPDRS scores and a 10–40 % reduction in on-period motor UPDRS scores with an associated 85 % reduction in l-dopa-induced dyskinesias [93]. In summary, STN DBS is quite effective in the treatment of Parkinson’s disease. Levodopa-responsive motor features typically respond well to STN DBS with the possible exception of tremor. The axial features of PD such as dysarthria, postural instability, and freezing of gait may not reliably respond to STN DBS. Disease-related progression of these features over time may compromise overall postsurgical benefit despite continuing response of limb motor function.
Preoperative levodopa responsiveness (by varying definitions) is often reported as a predictive factor for a positive response to surgery [12, 95–102]. A positive response to a levodopa challenge and correlation with good outcome may be more evident with STN DBS than with GPi DBS [103, 104]. The mean percent improvement in motor UPDRS during the levodopa test ranges from 40 to 70 % in published studies. As the levodopa response is the main predictor of outcome after STN DBS [96, 102, 103, 105], the benefit will be greatest in those patients who have a high off-drug score and a low on-drug score. In other words, the “best on” may be a better predictor for functional outcome than the numerical magnitude of the response.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree