PET and PET/CT of Malignant Melanoma
Hans C. Steinert
Malignant melanoma is the most aggressive cancer of the skin. The incidence of cutaneous malignant melanoma among whites living in the United States, Australia, and in Western Europe is increasing, but this increase is partly due to improved screening programs, although it seems undeniable the tumor is also occurring with greater frequency for other reasons (1). According to the American Cancer Society, malignant melanoma accounts for 1% to 2% of cancer deaths per year. As with most cancers, the causes of malignant melanoma are multifactorial. Numerous studies have demonstrated that the development and progression of melanoma are based on increasing levels of cutaneous solar exposure, especially to ultraviolet B radiation, in combination with the genotype, phenotype, and immunocompetence of the patient. The risk for a second melanoma is estimated to be about 5% in patients with a previous diagnosis of melanoma.
About 70% of melanomas are of the superficial spreading type. Nodular melanoma is the second most common type and comprises about 20% of melanomas. Lentigo maligna melanoma comprises 5% to 10%, and acral lentiginous melanoma 2% to 8% of melanomas. Cutaneous malignant melanoma can be located anywhere on the body, but lentigo maligna melanoma occurs primarily on the face, and acral lentiginous melanoma occurs primarily on the palms, soles, and nail beds. In women, melanomas most commonly occur on the lower extremities and in men on the back.
Any pigmented lesion with a change in size, configuration, or color should be considered a potential melanoma, and an excisional biopsy should be performed. Fortunately, in most developed countries, most patients are diagnosed early, so that melanoma can generally be cured by surgical excision of the lesion. Nevertheless, late diagnosis in locations that are not visible to the patient such as the scalp, the neck, the back, or in a plantar location are fairly common. The widely varying mortality reports in the literature depend more on the stage at diagnosis than on variations in surgical and treatment technique.
Histologic verification and accurate microstaging of tumor thickness are essential for treatment decisions and to predict the risk of metastases. For microstaging, two methods are used. The Breslow method measures the thickness of the lesion using an ocular micrometer to define the total vertical height of the melanoma, from its surface to the deepest part of the lesion. The Clark method categorizes different levels of invasion that reflect depth of penetration into the dermal layers and the subcutaneous fat (i.e., levels II, III, IV, or V). It has been demonstrated that the Breslow tumor thickness is the most important prognostic factor in clinically localized melanoma and is a highly reproducible parameter (2).
The American Joint Committee on Cancer (AJCC) has developed a four-stage system that allows subclassification of primary localized melanomas according to their malignant potential (3). Important prognostic factors, such as ulceration of the primary, microscopic or macroscopic nodal involvement, the number of positive nodes, the anatomic location of nodal, and distant metastases have been included in this staging system.
Stages I and II refer to localized melanoma and negative lymph nodes. Early malignant melanoma is curable by means of surgical excision. Stage III melanoma includes patients with lymph node metastases, either in regional nodes or as satellite or in-transit metastases. Patients with distant metastases represent stage IV. Despite the enormous progress of modern oncology, the prognosis of metastatic melanoma has remained particularly poor. Patients with regional lymphatic metastasis have a cure rate of approximately 20%, whereas no curative treatment is currently available for generalized metastatic melanoma, although occasional long-term survival has been reported for surgically resected oligometastatic disease.
The Breslow tumor thickness is an important prognostic factor in melanoma and guides treatment decisions. Patients with a Breslow tumor thickness up to 1 mm have an excellent prognosis not differing significantly from that of the general population. Patients with thin melanomas less than 1 mm are usually cured by excision of the primary lesion. Melanomas with a size of 1 to 2 mm are generally treated with wide local excision and are nowadays also commonly evaluated by sentinel lymph node scintigraphy. Patients with an indeterminate melanoma thickness of 2 to 4 mm have an increased risk of occult regional nodal metastases but have a relatively low risk (less than 20%) of distant metastases and are often staged using sentinel node detection methods. Melanomas greater than 4 mm in thickness have a 10-year survival of less than 40%. Once patients develop metastases, other prognostic factors have to be considered. The number of metastatic nodes has a significant prognostic value. Surgical excision of metastatic nodes is the only effective treatment for cure or locoregional disease control. These patients are generally treated by radical lymphadenectomy. High-risk patients with resected regional lymph node metastases may be candidates for various adjuvant chemo- or immunotherapies.
The high mortality of patients with melanoma is due to the early hematogenous spread. The mechanism of hematogenous spread and implantation of melanoma cells is poorly understood, and the location of metastases is unpredictable. The skin, subcutaneous tissue, and distant lymph nodes are the most common sites of distant metastases, but melanoma can metastasize to all organs. Early detection and surgical excision of single distant metastases are important in improving the prognosis. As soon as distant metastases are associated with a poor prognosis, significant factors predicting survival in patients with distant metastases are the number of metastatic sites and the remission duration (less than 12 months versus more than 12 months). The results of vaccine therapies for melanoma suggest that immune and clinical responses are promising in patients with metastatic disease.
For unknown primary melanomas, the distribution of metastases localized to a region or multiple sites at presentation is 43% and 57%, respectively. One must assume that the predominant origin of these unknown primary melanomas arises from a cutaneous melanoma that spontaneously regressed. Spontaneous regression is observed in up to 0.4% of melanomas and is likely mediated by an immune mechanism. The 5-year survival rate is similar to the rate of stage III disease (49%). Several reports have shown that the surgical management of patients with lymph node disease from unknown primary melanomas fared as well as patients with known cutaneous primary sites.
The clinical course of melanoma can be characterized by the risk of recurrent disease and death well beyond 10 years after the initial diagnosis. Twenty-five percent of the patients who survive more than 10 years will experience recurrence. Among patients with stage III disease, about 70% relapse, and two-thirds are at distant locations. Therefore, lifetime annual follow-up has been recommended, and imaging studies are a key to this follow-up.
Staging of Malignant Melanoma
Proper tumor staging is a key prerequisite for choosing the appropriate treatment strategy in melanoma. After resection of the primary melanoma, the analysis of Breslow tumor thickness gives an immediate estimate of the likelihood of regional lymph node metastases and distant metastases. In patients with thin melanomas (Breslow thickness less than 1 mm), the likelihood of metastases is so small that staging with imaging modalities is not cost-effective, although in some centers, for submillimeter lesions, sentinel nodal identification procedures are performed.
Patients with a Breslow thickness of 1 to 4 mm have an increased risk of occult regional nodal metastases but have a relatively low risk of distant metastases. Hafner et al. (4) has demonstrated that sentinel lymph node scintigraphy and biopsy is the most effective examination for staging these patients at baseline. One hundred consecutive patients with malignant melanoma and a tumor thickness greater than 1 mm were enrolled in the study. The patients underwent extensive baseline staging including physical examination, ultrasound of the regional lymph nodes and of the abdomen, sentinel lymph node scintigraphy and biopsy, chest x-ray film, and whole-body fluorodeoxyglucose (FDG) positron emission tomography (PET). Twenty-six percent of patients had a positive sentinel lymph node among 90% with microscopic disease. The macroscopic nodal metastases could be detected by physical examination, ultrasound, or PET. Therefore, in the author’s institution, a sentinel lymph node scintigraphy is performed at baseline for microscopic staging. A whole-body PET scan is performed to screen for distant metastases only when a metastatic sentinel lymph node is found. This observation of higher sensitivity of sentinel node imaging/histological assessment as compared to PET for small volume tumor has been shown by several groups.
Patients with melanomas more than 4 mm have a high risk (greater than 30% to 70%) of developing distant metastases. Due to the erratic pattern of distant metastases whole-body staging is recommended. In the past, a combination of conventional imaging modalities have been used for staging of malignant melanoma, such as chest x-ray, ultrasound, computed tomography (CT), magnetic resonance imaging (MRI), and bone scintigraphy. However, specific identification of tumor tissue is difficult with these methods. Cross-sectional imaging methods are generally used to evaluate a specific region, rather than the entire body. Diagnosis of a lymph node metastasis with cross-sectional imaging modalities is mainly based on size, choosing 1 to 1.5 cm in short-axis nodal diameter as a cutoff value between benign and pathologic lymph nodes. However, using these criteria, metastases in small nodes can be missed, and reactively enlarged nodes can cause false-positive results, limiting the value of the methods. CT often misses small metastases, particularly those in bowel and bone. CT is known to have a high rate of false-positive findings if applied as a screening method in patients with malignant melanoma (5).
Due to the limitations of morphological imaging modalities, several radiopharmaceuticals have been used in nuclear medicine to visualize metastases of melanoma. These include gallium-67-citrate (6), immunoscintigraphy with monoclonal antibodies (7), indium-111-pentetreotide (8), technetium-99m-methoxyisobutyl isonitrile (9), iodine-123-methyltyrosin (10), fluorine-18-fluoroethyltyrosine (11), iodine-123-iodobenzufuran (12), fluorine-18-fluoro-dopa (13), bromine-76-bromodeoxyuridine (14), and fluorine-18-fluorodesoxyuridine (15). However, these radiotracers did not offer any significant advantage in diagnostic sensitivity in systemic staging for metastases of malignant melanoma. False-negative scan results were common because of the poor sensitivity. These radiotracers were found to be suitable for systemic staging of melanoma only in exceptional cases. Several carbon-11–labeled radiopharmaceuticals have been used for experimental studies in malignant melanoma. Due to the short half-life of carbon-11, the clinical use of these tracers for whole-body staging remains limited.
Whole-body PET and PET/CT Imaging with Fluorodeoxyglucose
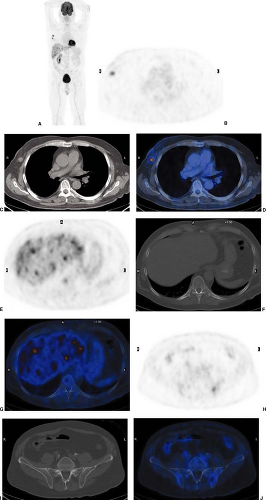
Today, 2-[18F]-fluoro-2-deoxy-D-glucose (FDG) is the most widely used radiopharmaceutical for staging of malignant melanoma. In vitro and in vivo experiments with tumor cells demonstrated higher FDG accumulation in melanoma than in any other tumors (16). Whole-body PET and integrated PET with CT (PET/CT) with FDG have proven to be highly effective and cost-saving modalities to screen for metastases of malignant melanoma throughout the body (17,18,19,20,21,22,23,24,25,26). With the exception of the brain and the lung, whole-body FDG PET and PET/CT scans largely replace the standard battery of imaging tests currently performed on high-risk patients (Fig. 8.11.1).
Due to the erratic pattern of metastases of malignant melanoma, whole-body FDG PET scanning or PET/CT scanning is
required. In the author’s institution, only combined PET/CT systems are available. A whole-body PET/CT scan is routinely performed from the head to the knees. If the primary tumor was located in a lower limb, additional PET/CT scanning is performed from the knee to the feet for the detection of satellite lesions or in-transit metastases (N3 stage).
required. In the author’s institution, only combined PET/CT systems are available. A whole-body PET/CT scan is routinely performed from the head to the knees. If the primary tumor was located in a lower limb, additional PET/CT scanning is performed from the knee to the feet for the detection of satellite lesions or in-transit metastases (N3 stage).
In the author’s institution, whole-body PET/CT with FDG is restricted to patients with high-risk malignant melanoma (Breslow tumor thickness greater than 4 mm or a known metastasis). The preselection of patients ensures the high effectiveness of PET/CT imaging. According to the guidelines of the Swiss Society of Dermatology, PET or PET/CT scanning is annually recommended in the first 5 years after the diagnosis of a high-risk melanoma (27). For baseline staging, sentinel lymph node scintigraphy and biopsy are routinely performed in patients with a Breslow tumor thickness from 1 to 4 mm. In cases of microscopic tumor spread to the sentinel lymph node, an additional whole-body PET/CT for screening of distant metastases will be performed.
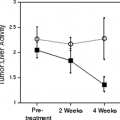
Whole-body FDG PET or PET/CT should be used to exclude unsuspected occult metastases in patients in whom surgery of a known metastasis is planned. If multiple metastases are present, chemotherapy is the therapy of choice. In extended disease, only palliative therapy is indicated. Whole-body FDG PET or PET/CT plays an important role in the evaluation of patients when immunotherapy is considered. Adjuvant treatment with recombinant interferon α is only indicated in disease-free patients after resection of high-risk melanoma. FDG PET or PET/CT is also useful in evaluating the treatment response.
As early as 1993, Gritters et al. (17) described promising results of FDG PET imaging in melanoma. In this initial study, the sensitivity of PET was 100% for intra-abdominal visceral and lymph node metastases, but it enrolled only 12 patients in the study with known metastatic melanoma on conventional staging. This study clearly demonstrated that melanoma generally has a high FDG accumulation, that PET can detect most lesions seen by conventional imaging with the exception of pulmonary nodules, and that PET also detects occult disease originally missed by CT while correctly excluding morphological false-positive lesions.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree