Hematologic malignancies are a broad category of cancers arising from the lymphoid and myeloid cell lines. The 2016 World Health Organization classification system incorporated molecular markers as part of the diagnostic criteria and includes more than 100 subtypes. This article focuses on the subtypes for which imaging with positron emission tomography/computed tomography (PET/CT) has become an integral component of the patient’s evaluation, that is, lymphoma and multiple myeloma. Leukemia and histiocytic and dendritic cell neoplasms are also discussed as these indications for PET/CT are less common, but increasingly seen in clinic.
Key points
- •
FDG-PET/CT is the imaging modality of choice for staging and assessing end of treatment response for FDG-avid lymphomas. Interim FDG-PET/CT has prognostic value, which has led to risk-adapted treatment strategies with many clinical trials evaluating escalation and de-escalation strategies.
- •
FDG-PET/CT can be used as an alternative to whole-body CT or whole-body MRI for evaluation of many plasma cell disorders and is the recommended modality of choice for assessing response to treatment in multiple myeloma.
- •
FDG-PET/CT can detect extramedullary disease in patients with leukemia although the prognostic relevance and applications for assessing response are unclear.
- •
FDG-PET/CT can detect sites of disease in patients with histiocytosis but the definitive role of FDG-PET/CT has yet to be defined.
- •
Many patients with hematologic malignancies use medications that lead to false-positive and false-negative results on FDG-PET/CT such as immunomodulatory drugs, steroids, and G-CSF, so it is important that the interpreter be aware of these potential pitfalls.
Introduction
Hematologic malignancies are a broad category of cancers arising from the lymphoid and myeloid cell lines. The 2016 World Health Organization classification system incorporated molecular markers as part of the diagnostic criteria and includes more than 100 subtypes. , This article focuses on the subtypes for which imaging with positron emission tomography/computed tomography (PET/CT) has become an integral component of the patient’s evaluation, that is, lymphoma and multiple myeloma (MM). Leukemia and histiocytic and dendritic cell neoplasms are also discussed because FDG-PET/CT for these indications, although less common, are increasingly being seen in clinic.
Normal anatomy and physiology
The lymphatic system comprises lymph nodes, lymphatic ducts, thymus, spleen, tonsils, and Peyer’s patches in the small bowel. The bone marrow is also considered part of the lymphatic system, but does not have any lymphatic drainage. Lymph nodes are bean-shaped structures of lymphatic tissue contained in a fibrous capsule that filter interstitial fluid from afferent vessels and return it to the circulation via efferent vessels. The major structural components of lymph nodes are as follows: (1) inner medulla layer; (2) outer cortex containing multiple germinal centers; and (3) inner cortical layer (paracortex) containing deep cortical units. B-lymphocytes (B-cells) are found in the cortex and medulla, whereas T-lymphocytes (T-cells) are in the paracortex. On imaging, normal lymph nodes have a smooth, well-defined borders, are less than 1 cm, and have a fatty hilum. The spleen is composed of many lobules, each containing peripheral red pulp and core white pulp, within a connective tissue sheath. The upper limit of normal for spleen size is 12 cm in maximum longitudinal dimension; however, emerging evidence suggests normal splenic size should be adjusted for sex and height.
The thymus is a bilobed, triangular-shaped primary lymphatic organ located in the anterior mediastinum and its major function is to mature and educate T-lymphocytes. The thymus is large in childhood, grows to peak size during puberty, and then begins involuting in early adulthood with progressive fatty infiltration, often with no residual thymic tissue visible on imaging in adults older than 40 years. The bone marrow consists of richly vascularized red marrow where hematopoiesis occurs and fatty yellow marrow containing inactive hematopoietic tissue. Red marrow predominates at birth occupying most of the skeleton. The normal adult distribution of marrow is reached at about 25 years of age with active red marrow persisting in the axial and proximal appendicular skeleton.
Protocol and imaging technique
The most commonly used radiotracer for PET/CT to evaluate hematologic malignancies is fluorine-2-deoxy-2-[ 18 F]fluoro- d -glucose ( 18 F-FDG). Detailed imaging protocols are found in the European Association of Nuclear Medicine and American College of Radiology procedure guidelines , Briefly, 18 F-FDG is injected intravenously followed by an uptake phase (goal 60 minutes, acceptable range 50–75 minutes), then sequential PET and CT images. 18 F-FDG activity administered ranges from 5 to 20 mCi (185–740 MBq) depending on the PET scanner, local acquisition and reconstruction protocols, and patient weight. CT protocols also vary from low dose for attenuation correction to fully diagnostic with intravenous contrast administration. In preparation for the scan, patients should fast 4 to 6 hours, avoid strenuous activity for 24 hours before the examination, and be well hydrated. It is generally recommended that blood glucose levels are less than 200 mg/dL immediately before injection of 18 F-FDG; however, some circumstances, like a research trial, might require stricter cut-offs.
Most patients with lymphoma have a standard PET/CT from the base of the skull through the mid-thigh, with exceptions including known sites of disease outside that range whereby the field of view can be tailored accordingly. A total body PET/CT is performed for patients with myeloma and for patients with leukemia requiring assessment for extramedullary disease.
Lymphoma
Classification
Lymphoma is classified into two major categories: non-Hodgkin lymphoma (NHL) and Hodgkin lymphoma (HL). NHL is the most common with more than 30 subtypes described based on the abnormal cell lineage of origin—B-cell, T-cell, or rarely natural killer cell (NK-cell). The most common subtypes of NHL in the USA are diffuse large B-cell lymphoma (DLBCL) and follicular lymphoma (FL). HL represents approximately 10% of all lymphoma diagnoses and is subcategorized as classical Hodgkin lymphoma (cHL) and lymphocyte-predominant Hodgkin lymphoma (lpHL, 5% of all HL).
Clinical Presentation and Natural History
NHL usually presents as painless lymphadenopathy with approximately 40% of patients reporting “B-symptoms” of fever, night sweats, and/or weight loss at diagnosis. The natural history of NHL is variable between and within each histologic subtype. DLBCL typically has a more aggressive course, whereas FL is usually considered indolent but can behave indolently or aggressively depending on the histologic grade.
NHL mostly involves lymph nodes, but extranodal sites of disease are more frequent in NHL compared with HL. The spleen is considered an extranodal site in NHL, but nodal in HL. The most common sites of extranodal disease include the stomach, spleen, Waldeyer’s ring, the central nervous system (CNS), lung, bone, and skin. The pattern of spread in NHL is less predictable than HL; however, there are some patterns that favor one entity over the other. For example, mediastinal disease is less common in NHL (∼20% of patients), whereas mesenteric or epitrochlear lymphadenopathy or involvement of Waldeyer’s ring is more common in NHL compared with HL.
HL also usually presents as painless supradiaphragmatic lymphadenopathy and about 30% of patients experience “B-symptoms.” Classical HL is considered as a more aggressive disease, whereas lpHL behaves more like a low-grade NHL. Roth and colleagues evaluated patterns of lymph node spread in 297 patients with HL who underwent pathologic staging by assuming either right or left cervical nodes as the primary site of origin as 70% of patients with stage I disease had involvement of cervical nodes. They found two distinct patterns of disease determined by laterality. In patients with right cervical nodes, the disease spreads first to the upper mediastinum, then pulmonary hilar nodes, upper abdominal nodes, and then to the spleen. In patients with left cervical nodes, the spread was directly to the upper abdomen bypassing the mediastinum, then back up to pulmonary hilar nodes, upper mediastinum, the contralateral cervical chain, and then on to axillary and finally inguinal nodes.
Clinical Applications of Imaging and Diagnostic Criteria: Diagnosis and Histologic Grading
Most lymphomas are FDG-avid. , In general, aggressive NHL is intensely FDG-avid, whereas the more indolent subtypes such as small lymphocytic lymphoma (SLL), enteropathy-type T-cell lymphoma, extranodal marginal zone lymphoma (MZL), mucosa-associated lymphoid tissue (MALT) MZL, lymphoid papulosis, and primary cutaneous anaplastic large T-cell lymphoma have lower levels of FDG uptake.
There is no set value defining high versus low levels of FDG uptake in NHL; however, Schoder and colleagues found that an SUV less than 10 identified 81% of indolent NHL and an SUV ≥13 indicated aggressive disease. FDG-PET/CT is not a replacement for histologic grading but may guide tissue sampling to areas of higher FDG uptake that may harbor more aggressive disease. This is especially important for identifying high-grade transformation in the setting of existing low-grade disease.
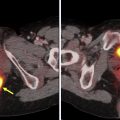
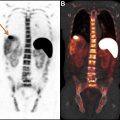
Transformation to a high-grade lymphoma may be seen in a variety of indolent NHL subtypes, including chronic lymphocytic leukemia (CLL)/SLL (ie, Richters transformation), FL, mantle cell lymphoma (MCL), and MALT, and is associated with poor outcomes. Several studies evaluated the use of FDG-PET/CT for identifying high-grade transformation. In patients with CLL, which typically has low-level homogeneous FDG uptake, the strength of FDG-PET/CT is its high negative predictive value of 97% using a cut-off point of SUVmax 5 to exclude Richter’s transformation. In a more heterogeneous group of patients with NHL including FL, CLL, Waldenstrom’s macroglobulinemia, and MZL and clinical concern for transformation, FDG-PET/CT was prospectively used to guide biopsy at the site of highest SUVmax; an SUVmax less than 11.7 was always associated with indolent lymphoma and SUVmax greater than 17 was always associated with high-grade transformation. The larger the gradient between the indolent lymphoma and potential site of transformation, the more specific FDG-PET/CT is for identifying high-grade transformation ( Fig. 1 ).

Both forms of HL are FDG-avid. A limited number of studies have suggested that lpHL may be less FDG-avid than cHL. , Results regarding differences in levels of uptake between cHL subtypes were mixed.
Clinical Applications and Diagnostic Criteria: Initial Staging and Treatment Response
FDG-PET/CT is the imaging modality of choice for the initial staging of FDG-avid lymphoma. FDG-PET/CT changes initial staging in 10% to 30% of patients compared to CT, although this does not necessarily result in a management change for all cases. The 2014 Lugano classification is the current system for staging and response assessment for lymphoma, and includes criteria for FDG-PET/CT as well as CT for non–FDG-avid lymphoma subtypes or when PET/CT is not available. The Lugano classification includes a modified Ann Arbor staging system, but current management strategies rely more on prognostic and risk factors than stage alone.
One major change in the Lugano classification is more judicious recommendations for using bone marrow biopsy as an initial staging procedure based on studies demonstrating that FDG-PET/CT is more accurate than standard iliac crest biopsy, particularly in HL and aggressive NHL subtypes. In the current Lugano classification, FDG-PET/CT obviates the need for bone marrow biopsy in HL, and bone marrow biopsy in DLBCL is only recommended in the setting of a negative FDG-PET/CT and the need to identify a discordant lymphoma subtype in the bone marrow where management would be altered by a positive result.
FDG-PET/CT is also now the modality of choice to assess response to treatment for FDG-avid lymphoma. This is based on the ability of PET to differentiate residual active tumor from post-treatment changes in residual mass on CT during and after treatment. End of therapy (EOT) FDG-PET/CT is routinely performed approximately 6 to 8 weeks after completion of first-line systemic therapy or 2 to 3 months or more after completion of radiotherapy to determine remission status. A positive EOT FDG-PET/CT portends a poor prognosis across most lymphoma subtypes and for DLBCL and HL, treatment is typically escalated in the setting of a positive EOT FDG-PET/CT scan, , although management changes are specific for different stages and risk factors and a complete review is beyond the scope of this paper. Interim FDG-PET/CT (iPET) is performed midway through treatment to determine chemosensitivity and early treatment response and provides prognostic information. Integration of how this information affects management has been more variable than EOT FDG-PET/CT and across lymphoma subtypes and is discussed further below.
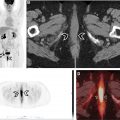
For both EOT and interim FDG-PET/CT, the 5-point Deauville score (DS) is used to assess metabolic activity in tumor compared to internal references of mediastinal blood pool and normal liver uptake by visual assessment , ( Figs. 2–4 , Table 1 ). A DS of 1 or 2 is considered a complete metabolic response. A DS of 3 is interpreted depending on the timing of the PET/CT, the clinical context, type of treatment, and whether the patient is on a clinical trial. A DS of 4 or 5 on iPET is considered a partial treatment response if the FDG uptake has decreased from the baseline study or treatment failure if unchanged or increased from baseline. On EOT PET/CT, a DS of 4 or 5 is considered a treatment failure.



| Deauville Score | Visual Assessment of Metabolic Activity on FDG-PET/CT |
|---|---|
| 1 | No uptake |
| 2 | Uptake less than or equal to mediastinal blood pool |
| 3 | Uptake greater than mediastinal blood pool and less than or equal to liver |
| 4 | Uptake moderately increased compared to liver |
| 5 | Uptake markedly increased compared to liver or any new site of uptake |
| X | Uptake not attributed to lymphoma |
The prognostic value of the DS on iPET has been validated in both HL and NHL with patients receiving a DS score of 1 to 3 (interpreted as a negative PET) having a better outcome than those with a DS of 4 or 5 (interpreted as a positive PET). This has led to risk-adapted therapy approaches based on iPET results and clinical trials have explored both escalation and de-escalation strategies and consider both efficacy and toxicity in regards to survival outcomes depending on disease stage and clinical prognostic factors.
In the RAPID trial, patients with early-stage HL and negative iPET after 3 cycles of adriamycin, vinblastine, bleomycin, and dacarbazine (ABVD) chemotherapy were randomly assigned to receive involved-field radiotherapy (IFRT) or no further treatment. A lower rate of progression and longer 3-year progression-free survival (PFS) was seen in the IFRT group, and the de-escalation strategy was not deemed noninferior to IFRT. Another important finding in the RAPID trial was that the IFRT group had a higher rate of death because of nonprogression raising the question regarding late toxicity from radiation therapy. A more recent meta-analysis showed decreased PFS in patients with early HL by omitting radiotherapy based on negative iPET; however, overall survival (OS) was not changed. The EORTC H10 trial investigated both escalation and de-escalation strategies in early-stage I/II HL depending on iPET results. Patients with a negative iPET after 2 cycles of ABVD were randomized to receive additional chemotherapy ± radiation therapy. The chemotherapy alone arm was closed early because of higher recurrence rates. In the iPET positive arm after 2 cycles of ABVD, patients randomized to the escalated to bleomycin, etoposide, adriamycin, cyclophosphamide, vincristine, procarbazine, and prednisone (BEACOPP) and IFRT arm had improved 5-year PFS compared with those randomized to ABVD + IFRT. The role of iPET response assessment was evaluated in advanced HL on the RATHL trial, which found that the omission of bleomycin for those with negative iPET resulted in less pulmonary toxicity but not significantly lower efficacy. Although the 2020 NCCN guidelines for HL recognize the potential value of iPET for some specific clinical scenarios and regimens in HL, caution is also advised that its role is not established for all clinical scenarios.
Similar trials investigating iPET in patients with NHL have also been performed; however, the predictive value of the iPET for residual disease and outcome in DLBCL has been more mixed because of high false-positive rates. The NCCN guidelines for DLBCL recommend against iPET for guiding therapy as well as confirmatory biopsies or follow-up imaging in the setting of a positive iPET and consideration of therapy change.
Semiquantitative measures on iPET using SUV have also been proposed as an alternative to the qualitative DS. Several studies have found that a decrease in SUVmax of 66% between baseline and iPET is an optimal cut-off to determine whether patients with DLBCL were good responders or poor responders and using this has also proved to be predictive of response. , , In these studies, iPET was performed after two cycles of chemotherapy and other studies have shown that when iPET is performed later, after 3 or 4 cycles, the predictive value is not superior to DS. , More recently, Schöder and colleagues reported that the change in SUVmax was predictive of OS in patients with DLBCL. Other studies have reported methods of using SUVmax of residual tumor expressed as a ratio to SUVmax of the normal liver parenchyma which also showed predictive value. , Hasenclever and colleagues created a semiautomatic quantification measure qPET, which functions as an extension of the DS using SUVpeak of residual tumor, SUVmean of liver and mediastinal blood pool in a pediatric population with HL, which has since been reproduced in adults. These semiquantitative and quantitative measures are being evaluated with the aim of reducing interobserver variability for interpretation, although for now, the DS is still the official recommendation for response assessment.
The use of FDG-PET/CT in lymphomas other than DLBCL and HL and for follow-up after primary response assessment was recently reviewed by Karls and colleagues, which serves to provide more details for these indications.
Multiple myeloma
Classification
Multiple myeloma represents ∼10% of hematologic malignancies and results from abnormal proliferation of malignant monoclonal plasma cells. Monoclonal gammopathy of undetermined significance (MGUS) and smoldering multiple myeloma (SMM) are asymptomatic premalignant precursors to MM. The rate of progression to MM is ∼1% per year for patients with MGUS and ∼10% per year for those with SMM. , The diagnostic criteria for MGUS and SMM are shown in Table 2 and both require the absence of a myeloma-defining event. Myeloma-defining events indicate end-organ damage caused by the malignancy and include hypercalcemia, renal failure, anemia, and osteolytic bone lesions. Updated diagnostic criteria now include the presence of at least one of the biomarkers of malignancy as a myeloma-defining event, including findings on advanced imaging (see Table 2 ). Solitary plasmacytoma is an early-stage malignancy in the plasma cell neoplasm spectrum and can occur in bone or be extramedullary and progress to MM. The risk of a solitary plasmacytoma progressing to MM is higher in the presence of minimal bone marrow involvement (20%–60% at 3 years) than no marrow involvement (∼10%). Throughout the spectrum of the plasma cell neoplasms, whole-body imaging is of the utmost importance as the presence or absence of lesions directly influences diagnosis.
| Monoclonal Gammopathy of Unknown Significance | Smoldering Multiple Myeloma | Multiple Myeloma | |
|---|---|---|---|
| Serum monoclonal protein | <3 g/dL | ≥3 g/dL | — |
| Urinary monoclonal protein | – | ≥500 mg/24 h | — |
| Clonal bone marrow plasma cells | <10% | 10%–60% | ≥10% or biopsy proven plasmacytoma |
| Myeloma-defining events | Absent | Absent | Hypercalcemia Renal insufficiency Anemia Lytic bone lesion on XR, CT, PET/CT a >5 mm or >1 focal marrow signal abnormality on MRI Clonal bone marrow plasma cells 20%–60% Serum FLC ratio ≥100 (if involved FLC ≥100 mg/L) |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree