PET in Breast Cancers
Farrokh Dehdashti
Breast cancer is the most common malignancy in women, accounting for approximately 26% of all female cancers. It is estimated that in the year 2008 that there will have been nearly 182,460 new cases of breast cancer and approximately 40,480 breast cancer-related deaths in the United States (1). Despite progress in early diagnosis and treatment, breast cancer remains a significant health problem. An increase in availability and advances in imaging techniques have the potential to improve the management of breast cancer. The past decade has witnessed the emergence of functionally based methods, such as positron emission tomography (PET), which have added new dimensions to the radiological evaluation of breast cancer.
Currently the role of imaging is not limited to the early detection of breast cancer, but it also involves staging and restaging, assessment of tumor behavior and prognosis, and monitoring response to therapy. This chapter focuses on the current and potential clinical applications of PET and PET combined with computed tomography (CT), as well as their limitations, and discusses how PET can help resolve some of the shortcomings of conventional imaging modalities in the management of patients with breast cancer.
Although a variety of biological and functional characteristics of breast cancer, such as blood flow and receptor status, can be studied with PET, the clinical applications are mainly focused on the assessment of glucose metabolism using 2-fluorine-18-fluoro-2-deoxy-D-glucose (FDG). This focus is related to tracer availability as well as the superior performance of FDG in tumor evaluation and characterization relevant to clinical management of cancer patients, and the fact that PET assessment of some of the biological features of breast cancer, such as tumor blood flow, do not appear to be clinically relevant based on present data.
Clinical and preclinical studies have shown increased FDG accumulation in breast cancer. Several specific tumor characteristics have been demonstrated to determine the degree of glucose metabolism. In human breast cancer, marked overexpression of the GLUT1 glucose transporter has been reported, and this is certainly an important factor contributing to the increased glucose accumulation within this cancer (2). Additionally, FDG uptake in breast cancer has been attributed to several other factors such as microvessel density, hexokinase activity, number of tumor cells/volume, proliferation rate (also reflected in necrosis), number of lymphocytes (not macrophages), and HIF1α for up-regulating GLUT1 (3,4).
The phosphorylation step may determine the uptake of FDG in breast cancer (5). In vitro studies of rat mammary tumors (RMT) and human breast cancer cells (MCF7 and HTB771P3) have demonstrated an inverse relationship between extracellular glucose levels and tumor FDG uptake (6,7). It also has been shown that in breast cancer cell line (MCF7), abrogation of p53 is associated with specific changes in glucose metabolism (8).
Imaging Protocol and Image Analysis
FDG PET imaging protocols differ from institution to institution; however, there are several important issues that should be considered for successful clinical FDG PET imaging of patients with breast cancer. In this group of patients, FDG should be administered intravenously in the arm opposite a suspected breast lesion or via a foot vein to avoid increased FDG uptake in normal axillary lymph nodes as a result of extravasation of FDG at the injection site. Patients should have been fasting for at least 4 hours prior to FDG injection and exercise should be avoided in the day prior to the PET scan.
Determination of blood glucose levels before FDG injection is recommended to identify patients with possible fasting hyperglycemia (7). In vitro studies of rodent and human mammary carcinoma cells in culture and in vivo studies in rodent tumors have demonstrated that acute hyperglycemia markedly impairs tumor FDG uptake (7). A significant decrease in tumor FDG uptake has been reported in the insulin-induced hypoglycemic state. Typically, patients are studied in the supine position; however, primary breast cancer may be visualized more clearly with the patient in the prone position using a scintimammography positioning pad, which provides less motion-related artifact and better separation of deep breast structures from the myocardium and liver and may decrease scatter from FDG uptake in these organs compared with that in the supine position (9).
It has been reported that the tumor-to-nontumor uptake ratio improves by obtaining delayed PET images 3 hours after administration
of FDG (10). Kumar et al. (11) reported that over time (average time interval of approximately 38 minutes between two scans), FDG uptake increased in breast cancer, whereas FDG uptake decreased in inflammatory lesions and normal breast tissues. They found that a percentage change of +3.75 or more in standard uptake value (SUV) over time is highly sensitive and specific in differentiating inflammatory lesions from malignant lesions. However, it is yet to be proven whether prone imaging or delayed imaging improves lesion characterization or detection sensitivity.
of FDG (10). Kumar et al. (11) reported that over time (average time interval of approximately 38 minutes between two scans), FDG uptake increased in breast cancer, whereas FDG uptake decreased in inflammatory lesions and normal breast tissues. They found that a percentage change of +3.75 or more in standard uptake value (SUV) over time is highly sensitive and specific in differentiating inflammatory lesions from malignant lesions. However, it is yet to be proven whether prone imaging or delayed imaging improves lesion characterization or detection sensitivity.
FDG PET imaging is performed with or without attenuation correction. No significant difference has been demonstrated in lesion detectability of attenuation-corrected or noncorrected images (10). Currently, as PET/CT replaces PET alone, fewer studies are performed without attenuation correction. It is recommended that both attenuation-corrected and noncorrected images be evaluated, as some breast lesions may be better seen on noncorrected images.
Although clinical oncologic FDG PET images are most often interpreted qualitatively, semiquantitative measurements offer more objective criteria for differentiating benign from malignant lesions and monitoring response to therapy. The most commonly utilized semiquantitative method is the SUV, also known as the differential uptake ratio or the differential absorption ratio. SUV is an index of glucose metabolism based on tissue uptake of FDG normalized to body weight (or lean body mass or body surface area) and injected dose (12,13). In the fasting state body fat has a much lower uptake of FDG than other tissues, so the SUVs of many tissues show a strong positive correlation with weight and may be overestimated in obese patients. This may be important when FDG PET studies performed before and after cancer treatment are compared to assess response to therapy, as SUVs may not be comparable if the patient loses a significant amount of weight between the two PET studies. It has been shown that SUV normalized to lean body mass or body surface area instead of total body weight is a more accurate estimate that will be less affected by changes in body weight (12,13).
Overall, in patients undergoing cancer therapy, efforts should be made to perform the studies in a similar fashion. Probably the most common source of variability in SUV determination is related to the duration of the uptake phase, as the SUV for most tumors continues to increase slowly over time, and no methods to correct for this variable are available.
The Patlak graphical approach also has been used in a limited fashion for quantitative evaluation of the FDG metabolic rate in breast lesions. This graphical approach provides an estimate of the net FDG phosphorylation rate constant (mL/min/g); this value is proportional to the glucose utilization rate based on the assumptions that the flow of tracer is unidirectional into the cellular compartment and follows first-order kinetics, and that it occurs only in an irreversible manner if the tracer is metabolized so that the metabolites are also irreversibly trapped (14). Although this type of analysis may provide a more reliable estimate of regional glucose metabolism, it is time consuming and technically demanding and therefore is not recommended for routine clinical studies of cancer patients who are often too ill to tolerate the lengthy imaging required.
A recent study demonstrated similar diagnostic accuracy for visual and semiquantitative (SUV and Patlak, respectively) analysis in differentiating benign from malignant breast lesions (15). The average SUV normalized to body weight and blood glucose and corrected for partial volume averaging had the highest diagnostic accuracy, exceeding that achieved with either the Patlak method or both average and maximum SUV uncorrected for blood glucose level and partial volume averaging (15).
Physiologic accumulation of FDG in normal breast tissue is variable, reflecting the amount of proliferative glandular breast tissue present. FDG uptake in normal breast is typically mild and diffuse with focally increased uptake in the areolar complexes. However, FDG uptake is more prominent in premenopausal women with abundant proliferative glandular breast tissue and in postmenopausal women undergoing hormone replacement therapy.
In a recent study of 96 patients with documented unilateral breast cancer, menstrual status or age was shown to have no effect on the degree of FDG uptake in the normal breast (16). Intense FDG uptake has been reported in lactating breasts, but not in postpartum breasts of women who are not breastfeeding. FDG is detected in minimal concentration in breast milk despite intense uptake in the breast itself. Therefore, radiation exposure to an infant is mainly due to close contact with the mother’s breast rather than to ingestion of radioactive milk. Interruption of breastfeeding for approximately 8 hours should be sufficient to keep the infant’s exposure below 500 mrem. However, radiation dose to the lactating breast can be substantial if breastfeeding is not terminated, and careful consideration must be given as to the diagnostic information available from the PET versus the increased radiation dose to the patient in such cases.
Detection of Breast Cancer
In randomized clinical trials, screening mammography has resulted in a reduction of breast cancer mortality by about 25% to 45% in women over the age of 40 (17). Recent significant progress in mammographic technique, such as computer-aided lesion detection, as well as the appropriate use of supplementary imaging techniques, such as ultrasonography and magnetic resonance imaging (MRI), have played an important role in the early detection of breast cancer.
Currently, the diagnosis of breast cancer is based on breast self-examination, physical examination by trained personnel, mammography supplemented as necessary by ultrasonography and MRI, and histological examination of identified breast lesions. Breast examination has low specificity and is not very sensitive for detection of small lesions, but is useful for detection of palpable breast masses. Mammography has a relatively high sensitivity, ranging from 54% to 81% depending on age, breast density, and menopausal status (18). The use of computer-aided lesion detection with mammography has resulted in an increase in the rate of cancer detection (19). Although mammography has a higher sensitivity for detecting breast cancer in fatty breasts, it is of limited value in several situations, such as detection of malignancy in dense breasts or postsurgical breasts (e.g., after breast augmentation, lumpectomy, or breast-conserving therapy for breast cancer), early detection of tumor recurrence after surgery, and monitoring response to therapy (20,21).
Mammography has a low positive-predictive value (10% to 35%) for nonpalpable cancers, and the frequency of positive biopsy findings after abnormal mammography ranges from 10% to 50% (22,23,24). Because of the low specificity of mammography, the majority of breast lesions are subjected to biopsy. Although the commonly employed fine-needle aspiration and stereotactic core biopsy are less invasive than excisional biopsies, they suffer from sampling errors.
Recently, MRI of the breast has attracted significant attention and, although the initial results in assessing mammographically difficult-to-examine breasts are very promising, its clinical role for general population screening has not yet been established. Currently, MRI is recommended for screening women at high risk for developing breast cancer. A recent review of the literature has shown that MRI mammography is a valuable adjunct to conventional imaging in the preoperative local staging of invasive breast cancer, in particular in the assessment of tumor extent, including detection of multifocal, multicentric, and bilateral disease (25).
PET imaging of the breast offers physiological information complementary to that achieved from conventional imaging techniques and, therefore, can be used to better characterize disease. However, PET has important limitations such as high cost, significant radiation exposure, and limited resolution, suggesting that the clinical use of PET should be reserved for a selected subset of women with breast cancer.
FDG uptake is considerably higher in malignant tumors than in normal tissues. Several studies have demonstrated that FDG PET has high sensitivity (64% to 96%) and specificity (80% to 100%) for detection of primary breast cancer and for differentiating breast cancer from benign lesions (26,27,28,29,30,31,32,33,34,35,36,37). In a study of 144 patients with breast masses, visual qualitative analysis of parametric (SUV normalized) FDG PET images was assessed. FDG PET images were evaluated using conventional image reading (CIR), in which only focal areas of markedly increased FDG accumulation were considered to represent malignancy, and sensitive image reading (SIR), in which those with diffuse or focal areas of moderately increased FDG accumulation were also considered to represent malignancy. Breast cancer was identified with a sensitivity of 64% and 80% using CIR and SIR, respectively. However, the increased sensitivity using SIR resulted in a significant decrease in specificity (94% to 75%) (32).
Avril et al. (32) also demonstrated that the diagnostic accuracy of FDG PET is dependent on tumor size. None of four stage pT1a tumors (less than 0.5 cm) and only one of eight stage pT1b tumors (greater than 0.5 to 1.0 cm) were classified as malignant lesions by FDG PET. However, FDG PET had high sensitivity (81% and 92% using CIR and SIR, respectively) for detection of pT2 breast cancers (greater than 2.0 to 5.0 cm).
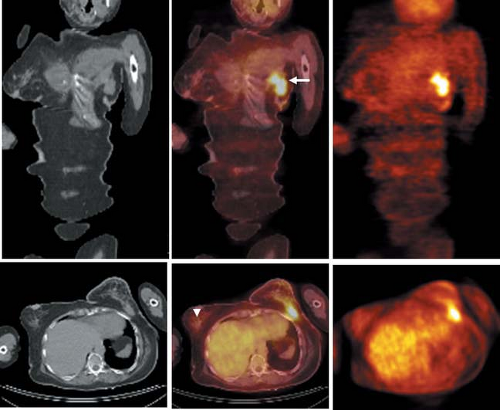
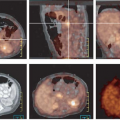
False-positive results have been reported in infectious and inflammatory lesions, including hemorrhagic inflammation after biopsy or surgery, due to accumulation of FDG in inflammatory cells (38,39). However, these conditions often can be easily recognized clinically. False-negative results are not limited to small lesions (typically less than 1 cm in diameter), but also are reported in slowly growing or well-differentiated tumors (such as tubular carcinomas, ductal carcinoma in situ, and lobular carcinomas) (27,32) (Fig. 8.12.1). The sensitivity of FDG PET for detection of breast cancer is also
dependent on the degree of uptake within the lesion in comparison to uptake in the surrounding tissue (5). It may be difficult to identify lesions with mild to moderate intensity against a background of diffusely increased activity, which is often seen in women with an abundance of proliferative glandular breast tissue (particularly young women). Therefore, FDG PET is not suitable for breast cancer screening due to its limited sensitivity for detection of small lesions, technical complexity, availability, and high cost, at least with currently available whole-body PET scanners.
dependent on the degree of uptake within the lesion in comparison to uptake in the surrounding tissue (5). It may be difficult to identify lesions with mild to moderate intensity against a background of diffusely increased activity, which is often seen in women with an abundance of proliferative glandular breast tissue (particularly young women). Therefore, FDG PET is not suitable for breast cancer screening due to its limited sensitivity for detection of small lesions, technical complexity, availability, and high cost, at least with currently available whole-body PET scanners.
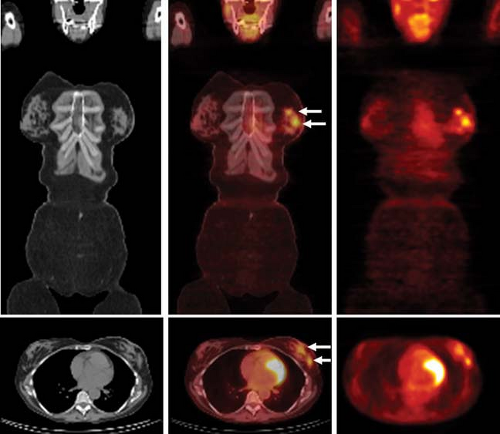
Recent technical advances that led to development of dedicated scanners specific for breast imaging may improve detection of small primary breast cancers by PET. One such device, a high-resolution PET scanner using mild breast compression (positron emission mammography [PEM]), consists of two moving detector heads, mounted on compression paddles, which perform volumetric acquisitions of the immobilized breast (40). PEM can be used as a stand-alone device or mounted on a stereotactic x-ray platform. In a recent study, Berg et al. (41) reported that PEM has sensitivity, specificity, positive-predictive value, negative-predictive value, and accuracy of 91%, 93%, 95%, 88%, 92%, respectively. When interpreted with mammographic and clinical findings PEM depicted 91% (10 of 11) ductal carcinoma in situ and 89% (33 of 37) invasive cancers. PEM was positive in one of two T1a tumors, four of six T1b tumors, and seven of seven T1c tumors. These scanners have the capability to coregister PET images and x-ray mammographic images.
The superior ability of PEM to depict early cancers compared to the whole-body PET scanners is likely a result of several factors, the capability to coregister PET images, better spatial resolution (1.5 mm FWHM vs. 4 mm), higher counting efficiency (leading to higher signal-to-noise ratios), and the use of modern iterative reconstruction techniques (42,43). The potential applications of this technique include evaluating mammographically difficult-to-examine breasts (i.e., radiodense breasts, postsurgical breasts), detecting an occult primary breast cancer in a patient with known axillary metastatic disease, and assessing the local extent of breast cancer (i.e., detecting multicentric or multifocal disease) (41) (Fig. 8.12.2). There may ultimately be a role for PEM as a supplemental screening tool considering the added functional information provided by this technique.
In untreated primary breast cancer, FDG uptake has been correlated with known prognostic factors by several investigators. This prognostic information provided by FDG PET may offer insight about the expected behavior of the tumor and guide the aggressiveness of the therapy. Strong positive correlations were found between FDG uptake (SUV) and several tumor characteristics, including histologic grade, cell proliferation indices, histologic type (with greater uptake in ductal cancers by comparison with lobular cancers), microvessel density, and number of tumor cells/volume (3,29,44,45,46,47).
No significant correlation has been reported between tumor FDG uptake and other known prognostic factors such as steroid-receptor levels (quantitative estrogen receptor [ER] and progesterone receptor [PR] concentrations], c-erb B2 expression, tumor size, presence of inflammatory cells, expression of GLUT1, percentage of necrotic, fibrotic, and cystic components, and the status of the axillary lymph nodes.
It has been shown that patient outcome may be predicted based on the degree of tumor FDG uptake (34,44,46,47,48). In one study, patients with a tumor differential absorption ratio SUV 3.0 or greater had a significantly worse overall survival (P <.0005) and disease-free survival (P <.0001) than those with tumor SUV less than 3.0 (45). In a multivariate analysis of known prognostic factors including tumor histologic grade, tumor size, number of positive lymph nodes, microvessel density, number of positive lymph nodes, and tumor FDG uptake assessed by SUV, FDG uptake was an independent predictor of disease-free survival (P = .0377). Similarly, Mankoff et al. (49) demonstrated that high tumor metabolic rate, as measured by FDG, relative to blood flow, as measured by [15O]-water, predicted poorer survival in patients with locally advanced breast cancer. The degree of FDG uptake within the primary tumor, as measured by peak SUV (SUVmax), has been shown to be predictive of survival. Inoue et al. (50) demonstrated that 5-year disease-free survival is less (75% vs. 95%) in patients with high FDG uptake than those with low FDG uptake (mean ± standard deviation 5.87 ± 3.12 vs. 4.02 ± 2.89; P = .011). In addition, the combination of high SUVmax and PET-positive axillary lymph node status was shown to be a highly significant risk factor being independent of the clinical T and N stages; patients with high SUVmax and PET-positive lymph node status showed a significantly (P <.001) poorer prognosis than the remaining patients (5-year disease-free survival of 44.4% vs. 96.8%).
These studies demonstrate that tumor FDG uptake provides relevant and unique information about tumor biology, which predicts tumor behavior and prognosis preoperatively before histopathological examination of the breast cancer. These data may assist in the selection of the most appropriate therapy for an individual patient.
Lymph Node Staging
The most common site of regional metastasis of breast cancer is the axillary lymph nodes. The status of the axillary lymph nodes is the single most important prognostic variable in the staging of early breast cancer and significantly influences the selection of treatment. Patients with histologically negative axillary nodes have significantly better survival rates than do patients with positive nodes, and survival among patients with positive nodes depends on the number of involved nodes (51,52). The 10-year survival rate in patients with histologically negative axillary nodes ranges from 65% to 80% versus 38% to 63% in those with involvement of one to three nodes and 13% to 27% in those with more than three positive axillary nodes (51). The likelihood of axillary nodal involvement is directly related to the size of the primary tumor at diagnosis (51).
Approximately 50% of patients with breast cancer evident on physical examination have histologic evidence of axillary nodal involvement (51). Clinical evaluation in predicting axillary nodal disease is inadequate, and, therefore, lymph node dissection (either conventional or more often limited with the use of sentinel node localization) is routinely performed to assess axillary nodal status. Currently the status of axillary lymph nodes is used to define patients in need of receiving axillary lymph node radiotherapy and also to determine the type and aggressiveness of adjuvant chemotherapy and hormonal therapy (53,54). Whether axillary nodal dissection also has therapeutic benefit for regional control of breast cancer remains controversial.
Currently, conventional or limited axillary nodal dissection, guided by lymphatic mapping using the sentinel lymph node technique, represents the standard of care in patients with invasive breast cancer. However, this procedure carries with it a relatively high cost and substantial morbidity. A noninvasive test that could reliably evaluate the status and extent of nodal involvement would be of great interest in the management of patients with breast cancer.
Accurate preoperative staging becomes even more important when one considers the increasing number of patients who are placed on neoadjuvant therapy prior to surgical removal of the primary tumor or lymph nodes. The surgical approach, including the role of sentinel node biopsy and delivery of radiation therapy after neoadjuvant therapy, in patients with breast cancer is evolving. Ongoing clinical trials will likely help to define which patients will benefit from neoadjuvant therapy and to determine the optimal multidisciplinary approach to the management of breast cancer.
If systemic neoadjuvant therapy prior to determination of the status of the axillary lymph nodes becomes routine, the histopathologic information about the number of involved axillary lymph nodes and the size of the primary tumor will no longer be available, and other methods will have to be identified to provide similar prognostic information. To address some of the limitations of such an approach, more recently the trend has been to assess the status of axillary lymph nodes prior to neoadjuvant therapy using the sentinel lymph node (SLN) technique or fine-needle aspiration with ultrasound or MRI guidance.
In preclinical studies of several types of tumors, Wahl et al. (55) demonstrated that FDG uptake in lymph nodes involved by metastatic tumor is greater than that in the normal lymph nodes. Although early studies suggested that FDG PET had the potential to be useful in noninvasive evaluation of locoregional nodal groups in patients with breast cancer and might reduce the number of patients requiring nodal sampling/dissection, more recent studies have been less encouraging (26,27,28,29,35,36,37,44,48,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71). The reported sensitivities have ranged from 20% to 100% and specificities from 66% to 100% for detection of axillary nodal metastasis. False-negative results with PET occur chiefly in patients with small deposits of tumors (typically less than 1 cm). The results of studies with 20 patients or more are listed in Table 8.12.1.
Based on the current data, a positive FDG PET may alleviate the need for a complete axillary nodal dissection because of its high specificity; however, a negative FDG PET does not preclude axillary nodal sampling or dissection given the high false-negative
rate. The PET detection of axillary nodal metastases is directly related to the size of the primary tumor and the number of lymph nodes involved. Avril et al. (35) demonstrated sensitivity and specificity of 94% and 100%, respectively, in patients with primary tumors greater than 2 cm in diameter and 33% and 100%, respectively, in patients with tumors less than 2 cm. Further, PET identified one of four patients with a single lymph node metastasis, four of six with two to five lymph node metastases, and all ten patients with more than five lymph node metastases. Schirrmeister et al. (37) demonstrated that FDG PET is superior to clinical evaluation for predicting axillary nodal involvement; the sensitivity and specificity were 79% and 92%, respectively, for FDG PET and 41% and 96%, respectively, for clinical evaluation of lymph nodes.
rate. The PET detection of axillary nodal metastases is directly related to the size of the primary tumor and the number of lymph nodes involved. Avril et al. (35) demonstrated sensitivity and specificity of 94% and 100%, respectively, in patients with primary tumors greater than 2 cm in diameter and 33% and 100%, respectively, in patients with tumors less than 2 cm. Further, PET identified one of four patients with a single lymph node metastasis, four of six with two to five lymph node metastases, and all ten patients with more than five lymph node metastases. Schirrmeister et al. (37) demonstrated that FDG PET is superior to clinical evaluation for predicting axillary nodal involvement; the sensitivity and specificity were 79% and 92%, respectively, for FDG PET and 41% and 96%, respectively, for clinical evaluation of lymph nodes.
Table 8.12.1 Fluorodeoxyglucose PET Results for Detection of Axillary Lymph Node Metastases | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Because of the limited resolution of PET, it is unlikely that this technique can detect small tumor deposits or determine accurately the number and pathological features of involved axillary nodes or extranodal spread. Thus, PET cannot replace axillary lymph nodal sampling, and treatment decisions should not be solely based on FDG PET results.
Axillary lymph node dissection is an important part of the surgical treatment of breast cancer. Axillary nodal dissection based on lymphatic mapping using SLN technique is an alternative to conventional complete axillary node dissection. The combined use of blue dye and lymphoscintigraphy gives the best SLN identification rate. SLN biopsy combined with immunohistochemical staining for cytokeratin to identify microscopic tumor foci accurately detects metastatic disease in lymph nodes and, thus, negative results identify patients who do not require complete axillary dissection. Several studies have compared FDG PET with histological results from SLN mapping and demonstrated low sensitivity, but high specificity of FDG PET for detection of axillary metastatic disease in early breast cancer (62,66,68,69,71,72,73) (Table 8.12.2). The high specificity may indicate that a complete axillary dissection without the need for SLN mapping should be performed in patients with positive PET.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree