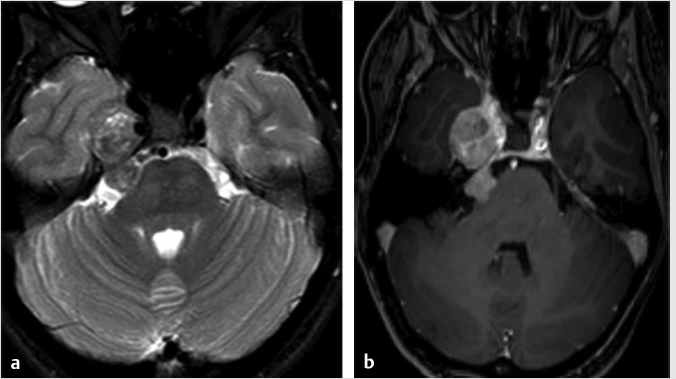
4 Petroclival and Lateral Skull Base The petroclival region can be defined as the region that includes the upper clivus and the anterior third of the petrous pyramid anterior to the internal acoustic meatus. It can be considered more as a surgical entity rather than an anatomical one, primarily due to the predilection of specific diseases such as petroclival tumors. The petroclival region or parasagittal skull base incorporates the greater sphenoid wing, cavernous sinus, branches of the trigeminal nerve, and the petroclival synchondrosis. The lateral skull base includes the far lateral aspect of the greater sphenoid wing, the lateral temporal bone, and the temporomandibular joint. When tumors affect the clival dura mater, they may also spread to the cavernous sinus, Gasserian ganglion, sphenoidal sinus, the sella, tentorial incisura, the porus, and the ventral edge of the foramen magnum. A lateral transpetrosal approach to the petroclival region is favored when exposure is needed beyond the lateral cavernous carotid and, even more so, the petrous apex and the Gasserian area. This lateral approach creates considerably broader exposure than other approaches and is also associated with reduced cerebral retraction. Since this region contains critical neurovascular structures, it is important to evaluate the anatomy accurately with noninvasive imaging prior to any surgical intervention.1,2,3,4,5,6,7,8 Given the complex and intricate anatomy of the skull base, high-resolution cross-sectional imaging is the mainstay for evaluating tumors in the petroclival region and lateral skull base. It is important to realize that computed tomography (CT) and magnetic resonance imaging (MRI) are complementary. Both of these modalities can help narrow the differential diagnosis based on evaluation of the site of origin, growth pattern, and the imaging features.9 Cross-sectional imaging also provides an assessment of the extent and accurate delineation of tumor margins, and the precise relationship between the tumor and important surrounding structures.9 MR imaging is the gold standard to assess the soft-tissue components, as it can routinely and accurately depict the intracranial extent of tumor. Specifically, it is helpful in evaluating for dural or leptomeningeal involvement, brain parenchymal invasion, and whether there is perineural tumor spread.9,10,11 Both CT and MRI are useful in evaluating osseous involvement, but CT is the gold standard to define the complex anatomy of the skull base and illustrate destructive change to the thin cortical margins of the skull base neural foramina, depict calcification, and assess bony growth rate and aggressiveness.9,10,12 The more permeative and erosive bone changes frequently indicate aggressive neoplastic lesions, while bone expansion, remodeling, and cortical thinning usually indicate a more benign indolent neoplastic lesion.9,12 It is important to remember that infectious processes can mimic both bone patterns. The typical MR protocol to image the skull base will require the three orthogonal imaging planes utilizing T1- and T2-weighted images, and gadolinium-enhanced T1-weighted images with and without fat suppression. Gradient echo (T2*) imaging is useful to detect the presence of absence of paramagnetic substances, including calcification, blood degradation products, and melanin.11,13,14 A high-resolution heavily T2-weighted sequence performed in the steady-state equilibrium will illustrate the relationships between the tumor and cisternal segments of the adjacent cranial nerves.10,12 The conventional spin echo T1-weighted sequence can provide information on bone marrow invasion with loss of the normal fat.11 If not contraindicated, administration of gadolinium contrast agents is essential to delineate the tumor extent and invasion as the tumor usually will demonstrate maximal enhancement signal and the other surrounding structures will enhance to a lesser degree.10,11,14 Additional useful information is provided with utilization of fat suppression to increase the conspicuity of the enhancement on postcontrast T1-weighted sequences. However, depending on the technique, it is important to be mindful of the pitfalls with fat suppression. For example, using frequency selective fat suppression techniques, there can be failure or inadequate fat suppression along the multiple interfaces in the skull base yielding false-positive results.9,12,15 This pitfall has increased the use of inversion recovery sequences (STIR) or Dixon-based fat suppression. Increased susceptibility effects can be seen at the skull base due to multiple air–soft tissue interfaces which has the potential for obscuring neural foramina; this can be mitigated to some extent by employing parallel imaging. Diffusion-weighted imaging provides high sensitivity to detect impeded water proton movement, which can be helpful to identify areas of increased tumor cellularity. As tumors can involve the intracranial compartment, additional whole brain evaluation with axial FLAIR and axial postcontrast sequences are also commonly employed. Utilization of parallel imaging and high field strength (3 tesla and beyond) can help in improved visualization of the finer details of the cranial nerves and cavernous sinus anatomy. The typical CT protocol on a multidetector CT involves acquisition of the images at 0.625 mm that allows reconstructions in all three orthogonal planes with isotropic resolution. It is important to reconstruct the images using both a soft tissue and a bone algorithm to be able to depict the complex bony anatomy and detect small areas of cortical thinning or destruction. As lesions in the petroclival region and lateral skull base are better depicted using MRI, contrast-enhanced CT is typically employed for preoperative planning and not for purely diagnostic reasons. 18 F-fluorodeoxyglucose positron emission tomography is very helpful in the posttreatment follow-up imaging protocol but plays no role in the initial diagnosis or evaluation of skull base tumors. Catheter angiography is primarily used to assess the patency of the circle of Willis when carotid artery sacrifice is being considered. While CT angiography may be sufficient for the initial workup of vascular anatomy, catheter angiography provides a dynamic way of assessing collateral flow and performs test occlusions. When appropriate, preoperative embolization of certain highly vascular tumors has the potential for reducing surgical mortality and morbidity.9,10,16 Meningiomas occur throughout central nervous system with about 2% occurring in the petroclival region. They are most commonly found in women around the age of 50 years.17 Although centered in the petroclival region, they can exert mass effect or compromise the surrounding structures due to the various sizes and configurations. These structures include the internal auditory canal (IAC), Meckel’s cave, cavernous sinus, jugular foramen, parasellar region, foramen magnum, and brainstem. They are almost always well-defined and circumscribed masses abutting the dura. As with most meningiomas, they are typically hyperattenuating on CT with about 25% containing calcifications.17 The calcifications may be diffuse, focal, globular, or “sunburst” in appearance. It is not uncommon to see hyperostosis on CT which accounts for 20 to 40% of cases.17 A majority of these lesions display intense homogenous enhancement on CT. When they grow large enough, they could demonstrate necrosis or cystic change, but hemorrhage is rare within the lesions. On MR, wherever possible, it is important to detect the cerebrospinal fluid (CSF) or vascular cleft between the tumor and brain parenchyma to ensure it is extra-axial in location. Up to 80% of meningiomas may demonstrate an enhancing “dural tail.”17 It is important to realize that the “dural tail” is not specific as it may be seen with other lesions such as a metastasis or schwannoma. They are isointense on T1-weighted images, hyperintense on T2-weighted images, and display avid homogenous enhancement unless there is necrotic or cystic change. Heavily calcified meningiomas may exhibit T1 and T2 hypointensity with variable enhancement, sometimes termed as a burned-out meningioma. The FLAIR sequence is useful to detect the perilesional reactive edema/gliosis in the surrounding parenchyma, while the gradient recalled echo or T2*sequence can illustrate the calcification. When clinically indicated, a MR venography can evaluate the dural venous sinus for invasion, thrombus, and/or occlusion. Catheter angiography is reserved for preoperative embolization which can reduce blood loss.17 The angiography will demonstrate a “sunburst” or radial appearance of arterial opacification within the tumor with the classic prolonged capillary “stain.” Schwannomas and neurofibromas can affect any cranial nerve. In the petroclival region, the trigeminal nerve is the most common affected nerve. Schwannomas are well-defined soft-tissue lesions which are hypointense on T1-weighted images and intermediate to hyperintense on T2-weighted images.18,19 They may have homogenous or heterogeneous enhancement after gadolinium administration ( Fig. 4.1 Axial T2-weighted (a) and postcontrast T1-weighted (b) images in a 45-year-old female patient demonstrate an enhancing lesion with heterogeneous signal on the T2-weighted image that involves the cisternal segment of the right trigeminal nerve and extends anteriorly over the petrous apex into the right middle cranial fossa. This was pathologically proven to be a trigeminal schwannoma. Paragangliomas, also known as glomus tumors, can include the jugular foramen and vagus nerve. The paragangliomas that develop along the jugular foramen wall are called glomus jugulare, while the ones that develop along the vagus nerve are called glomus vagale. When they occur at the cochlear promontory, they are called glomus tympanicum and arise from the paraganglia along the Jacobson nerve. Their MR features include hyperintense signal on T2-weighted images mixed with T2 hypointense foci (“salt and pepper” appearance) with avid enhancement ( Chondroid tumors including chondromyxoid fibroma and chondrosarcomas occur from the cartilaginous remnants at the petroclival synchondrosis.13,22 These chondroid tumors contain a typical chondroid matrix of arc and ringlike calcifications which are best seen by CT.22,23 On MR imaging, they demonstrate very high hyperintense signal on T2-weighted images which is a distinctive feature and are of low to intermediate signal on T1-weighted images ( Among all the neoplastic skull base lesions, metastasis is the most common tumor in the adult population.12 They are usually within the marrow space due to the increased vascularity. Common primary malignancies include lung, breast, prostate, and kidney.10,16 The CT and MR appearances can be quite variable; hence, diffuse bony metastatic disease can be confused with Paget’s disease or other fibro-osseous disease. The more hypervascular metastases can be confused with hypervascular primary lesions, including paragangliomas, hemangiopericytomas, and meningiomas. Metastasis can also present as perineural spread from squamous cell, adenoid cystic, and mucoepidermoid carcinomas.18 A plasmacytoma-related multiple myeloma is an expansile and destructive lytic lesion that occurs in the skull base but more commonly midline in location.13,16 They are hyperattenuating on CT and demonstrate intermediate signal on T1- and T2-weighted images.13,16 This is typical for hypercellular tumors with small round cells and a high nuclear–cytoplasmic ratio.13,16 Cutaneous malignancies such as squamous cell carcinoma ( While tumors are not uncommon entities in this region of the skull base, they have to be distinguished from nontumor entities such as infection, fibroosseous abnormalities, and Paget’s disease. Appropriate history of a short clinical course with imaging clues of bone destruction and significant periosseous enhancement are clues toward making a diagnosis of skull base osteomyelitis (
4.1 Surgical Anatomy of the Petroclival Region and Lateral Skull Base
4.2 Imaging Modalities for Evaluation of Petroclival and Lateral Skull Base Lesions
4.3 Neoplastic Pathology of the Petroclival and Lateral Skull Base Regions and Their Imaging Features
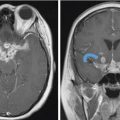
 Fig. 4.1).18,19 The larger tumors can show peripheral cysts and necrotic areas.18,19 These usually present as a single lesion but when encountering multiple schwannomas, it is important to consider neurofibromatosis type 2 or schwannomatosis.13 Neurofibromas are also well-defined soft-tissue lesions which are hypointense on T1-weighted images and intermediate to hyperintense on T2-weighted images.18,19 After gadolinium administration, they demonstrate avid homogenous enhancement.18,19 As schwannomas and neurofibromas grow along the nerve, they produce smooth enlargement of the nerve and exhibit spread both antegrade and retrograde along the trigeminal nerve.18,19,20 There can be focal or segmental enlargement with skip areas.20 These are usually benign; however, malignant schwannomas and neurofibromas exist and are most commonly seen in patients with neurofibromatosis type 1.13,19 The more malignant tumors are more aggressive and infiltrative lesions.16,19 While distinction between schwannomas and neurofibromas may not always be possible, the former are more frequently associated with hemorrhage, cyst formation, and fatty degeneration and are more eccentric and rounded, as opposed to the latter which are commonly fusiform and concentric.
Fig. 4.1).18,19 The larger tumors can show peripheral cysts and necrotic areas.18,19 These usually present as a single lesion but when encountering multiple schwannomas, it is important to consider neurofibromatosis type 2 or schwannomatosis.13 Neurofibromas are also well-defined soft-tissue lesions which are hypointense on T1-weighted images and intermediate to hyperintense on T2-weighted images.18,19 After gadolinium administration, they demonstrate avid homogenous enhancement.18,19 As schwannomas and neurofibromas grow along the nerve, they produce smooth enlargement of the nerve and exhibit spread both antegrade and retrograde along the trigeminal nerve.18,19,20 There can be focal or segmental enlargement with skip areas.20 These are usually benign; however, malignant schwannomas and neurofibromas exist and are most commonly seen in patients with neurofibromatosis type 1.13,19 The more malignant tumors are more aggressive and infiltrative lesions.16,19 While distinction between schwannomas and neurofibromas may not always be possible, the former are more frequently associated with hemorrhage, cyst formation, and fatty degeneration and are more eccentric and rounded, as opposed to the latter which are commonly fusiform and concentric.
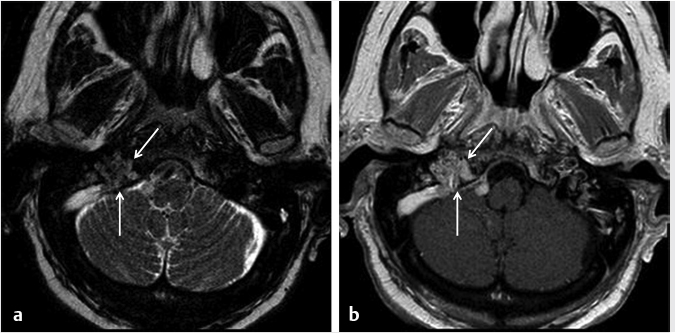
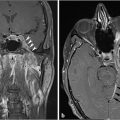
 Fig. 4.2).21 On CT, there can be permeativedestructive bony changes, particularly at the jugular foramen which distinguishes these tumors from meningiomas.21 Glomus jugulare tumors characteristically cause dehiscence of the hypotympanum of the middle ear. Most occur as a solitary lesion and are considered benign, despite their well-known predilection for invasion of adjacent structures. The lesions are considered malignant if metastases are identified associated with the lesion. On imaging, there are no reliable imaging features to distinguish the malignant from the benign forms other than identification of a metastatic lesion.21 MR and digital subtraction angiography is used preoperatively to assess the collateral cerebral blood flow and contralateral sigmoid sinus and internal jugular vein if the ipsilateral vessels will need to be sacrificed.21
Fig. 4.2).21 On CT, there can be permeativedestructive bony changes, particularly at the jugular foramen which distinguishes these tumors from meningiomas.21 Glomus jugulare tumors characteristically cause dehiscence of the hypotympanum of the middle ear. Most occur as a solitary lesion and are considered benign, despite their well-known predilection for invasion of adjacent structures. The lesions are considered malignant if metastases are identified associated with the lesion. On imaging, there are no reliable imaging features to distinguish the malignant from the benign forms other than identification of a metastatic lesion.21 MR and digital subtraction angiography is used preoperatively to assess the collateral cerebral blood flow and contralateral sigmoid sinus and internal jugular vein if the ipsilateral vessels will need to be sacrificed.21
 Fig. 4.3).22,23 The MR signal of the calcifications is variable depending on the size and calcium crystal composition.22,23 MR imaging of the skull base routinely includes fat-saturated sequences to define the tumor margins. Chondromyxoid fibroma is rare but presents as an expansile, noninfiltrating mass.24 These can demonstrate ground-glass density on CT.24 Chordomas and plasmacytomas occur at the skull base but are typically more midline in location. Chondroblastoma or chondromatous giant cell tumor is very rare but occasionally can originate in the lateral skull base.25
Fig. 4.3).22,23 The MR signal of the calcifications is variable depending on the size and calcium crystal composition.22,23 MR imaging of the skull base routinely includes fat-saturated sequences to define the tumor margins. Chondromyxoid fibroma is rare but presents as an expansile, noninfiltrating mass.24 These can demonstrate ground-glass density on CT.24 Chordomas and plasmacytomas occur at the skull base but are typically more midline in location. Chondroblastoma or chondromatous giant cell tumor is very rare but occasionally can originate in the lateral skull base.25
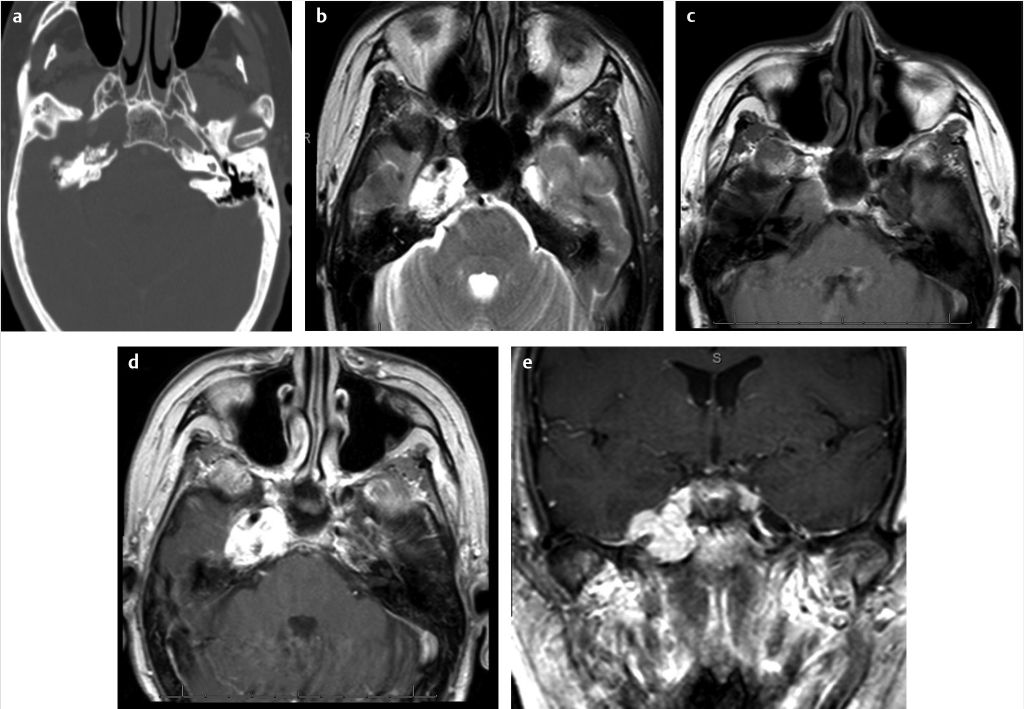
 Fig. 4.4) and basal cell carcinoma can also invade the underlying temporal bone and necessitate large surgical resection with or without radiation therapy. Endolymphatic sac tumors are rare tumors that have high association with Von Hippel– Lindau disease, especially when they are bilateral. Their location along the posterior aspect of the petrous temporal bone along with a moth-eaten appearance on CT is an important clue toward the diagnosis (
Fig. 4.4) and basal cell carcinoma can also invade the underlying temporal bone and necessitate large surgical resection with or without radiation therapy. Endolymphatic sac tumors are rare tumors that have high association with Von Hippel– Lindau disease, especially when they are bilateral. Their location along the posterior aspect of the petrous temporal bone along with a moth-eaten appearance on CT is an important clue toward the diagnosis ( Fig. 4.5).
Fig. 4.5).
 Fig. 4.6) or necrotizing otitis externa; however, tissue diagnosis may need to be obtained when the clinical and imaging pictures are indeterminate. Fibrous dysplasia has a characteristic ground-glass appearance on CT (
Fig. 4.6) or necrotizing otitis externa; however, tissue diagnosis may need to be obtained when the clinical and imaging pictures are indeterminate. Fibrous dysplasia has a characteristic ground-glass appearance on CT ( Fig. 4.7), while Paget’s disease typically presents with a mixed lytic-sclerotic appearance with or without coarsened trabeculae, thickened cortices, and bone hypertrophy in late stages (
Fig. 4.7), while Paget’s disease typically presents with a mixed lytic-sclerotic appearance with or without coarsened trabeculae, thickened cortices, and bone hypertrophy in late stages ( Fig. 4.8). The imaging characteristics of common neoplastic pathologies seen in these locations are summarized in
Fig. 4.8). The imaging characteristics of common neoplastic pathologies seen in these locations are summarized in  Table 4.1.
Table 4.1.
Radiology Key
Fastest Radiology Insight Engine