Introduction
The pharynx is a fibromuscular tube situated directly anterior to the vertebral column. It extends from the skull base to the lower border of the cricoid cartilage. It comprises a group of six muscles that are predominantly responsible for the voluntary act of swallowing: three pharyngeal constrictor muscles (superior, middle, and inferior constrictor muscles) and three vertically oriented muscles (stylopharyngeus, salpingopharyngeus, and palatopharyngeus). They are innervated by the pharyngeal branch of the vagus nerve (cranial nerve [CN] X), with the exception of the stylopharyngeus muscle, which is innervated by the glossopharyngeal nerve (CN IX). Based on the location, the pharynx is often subdivided into the following three sections:
- •
Nasopharynx: top of the pharynx, located behind the nasal cavity and extends from the skull base to the soft palate
- •
Oropharynx: located behind the oral cavity and extends from the uvula to the hyoid bone
- •
Hypopharynx: extends from the hyoid bone to the cricopharyngeus muscle, located at C5-C6 level, at the lower end of the cricoid cartilage
Imaging Rationale and Techniques
Computed tomography (CT) and magnetic resonance imaging (MRI) are currently the primary modalities for investigating pathologies of the pharynx. Although the superficial extent of mucosal lesions can be readily identified by the examining physician, their deep submucosal extent is almost totally inaccessible to clinical or endoscopic evaluation. CT offers rapid image acquisition and is helpful in the acute setting for rapidity of acute diagnosis. In particular the high spatial resolution of CT can provide precise anatomic detail. MRI on the other hand offers benefits over CT because of its higher soft tissue contrast resolution, multiplanar capability, and superiority in detecting perineural tumor spread and intracranial invasion. The role of advanced biological imaging techniques such as MR spectroscopy, MRI diffusion and perfusion, CT perfusion, and fluorodeoxyglucose positron emission tomography (FDG-PET) is discussed toward the end of this chapter.
Nasopharynx
Anatomy
The nasopharynx is the superiormost portion of the pharynx, located immediately below the skull base. It is attached to the undersurface of the clivus by the pharyngobasilar fascia of the superior constrictor muscle. The posterior and lateral walls of the nasopharynx are in continuity with the posterior and lateral oropharyngeal walls, respectively, and it is inferiorly limited by the soft palate.
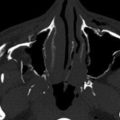
Along the lateral nasopharyngeal wall there are two indentations and one protrusion into the lumen. The indentations (recesses) are the eustachian tube orifice and the lateral pharyngeal recess (often known by its eponym, the fossa of Rosenmüller ), whereas the protrusion is the torus tubarius (the cartilaginous portion of the eustachian tube). On axial images, the lateral pharyngeal recess is located posterior to the torus tubarius, and it is superior to the torus tubarius on coronal images ( Fig. 22-1A-B ). The posterior pharyngeal wall has an undulating contour from the prevertebral longus capitis muscles, which demarcates the posteromedial aspect of the lateral pharyngeal recess.

There is a small lateral hiatus or opening in the skull base attachment known as the foramen of Morgagni through which the eustachian (pharyngotympanic) tube and levator veli palatini muscle pass (see Fig. 22-1C-D ). The levator veli palatini and the tensor veli palatini muscles are identifiable on T1-weighted MRI scans in the lateral wall of the nasopharynx and are responsible for opening the eustachian tube and for tensing and elevating the palate to prevent oronasal reflux. The eustachian tube communicates with the middle ear, allowing both middle ear aeration and drainage. The foramen of Morgagni is an essential anatomic structure but is also a potential weak spot through which nasopharyngeal neoplasms or infections may spread directly to the skull base or laterally to the parapharyngeal fat.
Congenital Lesions
Thornwaldt’s Cyst.
Thornwaldt’s cyst, named after German physician Gustav Ludwig Thornwaldt, is a common developmental benign midline nasopharyngeal mucosal cyst. It is usually asymptomatic and is detected incidentally on imaging. Peak incidence has been variably reported between the ages of 15 and 60 years. It has an autopsy prevalence of approximately 4%, with no gender predilection. It results from focal adhesion of pharyngeal mucosa to the notochord (a midline collection of cells that aids embryonic long-axis and neural plate development), which is then carried up as the notochord ascends to the developing skull base. This creates a potential space known as the pharyngeal bursa, whose orifice becomes obliterated after an attack of pharyngitis, thereby forming the cyst. A Thornwaldt’s cyst lacks osseous involvement and corresponds to a superior level in the clivus. These are almost always asymptomatic; however, if they become infected the patient presents with persistent or periodic nasopharyngeal drainage, halitosis, and foul taste. Some may present with otitis media due to obstruction of the eustachian tube. Dull occipital headache has also been described. A symptomatic cyst is also called Thornwaldt’s disease.
Thornwaldt’s cyst appears as a well-circumscribed, thin-walled, midline (but may be slightly paramidline) posterior nasopharyngeal mucosal space cyst lodged between the prevertebral muscles. It varies in diameter from a few millimeters to several centimeters. On CT scans, a small cyst may be missed, but a larger one usually is seen as a hypodense fluid-attenuation lesion ( Fig. 22-2A ). CT attenuation of the cyst increases, as does its protein content, and occasionally it may mimic a soft tissue mass. On MRI the T1-weighted signal intensity increases from low to high with increasing protein content. The cyst is bright on T2-weighted (see Fig. 22-2B ) and fluid-attenuated inversion recovery (FLAIR) images, with thin peripheral enhancement on postcontrast images.

Persistent Canalis Basilaris Medianus.
Persistent canalis basilaris medianus (CBM) is a rare and typically asymptomatic congenital anatomic variant of the basiocciput, believed to arise from notochordal remnants. It occurs in approximately 2% to 3% of adults as a well-defined corticated tubular structure, usually over 2 mm in diameter, originating on the intracranial surface of the basiocciput in close proximity to the anterior rim of the foramen magnum ( Fig. 22-3 ). It is thought to represent an embryologic remnant demarcating the cephalic end of the notochord and corresponds to the course of the notochordal canal in the caudal basiocciput. A complete CBM traverses entirely through the basioccipital bone, whereas an incomplete CBM can extend partially through either the cranial or pharyngeal portions of the basiocciput but does not extend all the way through to the other surface. Clival defects from this vestige can be associated with several anomalies. MRI may detect accompanying cysts and help differentiate between other possible etiologies, such as a cephalocele, and ecchordosis physaliphora, which is usually intradural within the prepontine cistern and is attached to the dorsal wall of the clivus via pedicles.

Fossa Navicularis.
Fossa navicularis is a notchlike bone defect in the basiocciput ( Fig. 22-4 ) and is also an incidental imaging finding. Its incidence is around 3%, with many being less than 2 mm in size. Rarely, lymphoid tissue can be found within a prominent fossa navicularis, what some authors have termed fossa navicularis magna. Transmission of infection from oropharyngeal soft tissues via the fossa navicularis has been rarely reported as a cause of clival osteomyelitis, raising the possibility of affected patients being more susceptible to other infections such as meningitis. It is important to be aware of this rare congenital variant to prevent mischaracterization with the many more worrisome possibilities and possibly avoid biopsy or unnecessary treatment attempts.

Craniopharyngeal Canal.
The craniopharyngeal canal (CPC) is a rare well-corticated defect through the midline of the sphenoid bone, extending from the floor of the sella turcica to the roof of the nasopharynx ( Fig. 22-5 ). The CPC is postulated to arise from an error in the normal development of the pituitary gland. It is a rare but important entity to recognize in the evaluation of nasopharyngeal or midline skull base lesions, because correct diagnosis may indicate pituitary dysfunction and obviate the need for surgery, thus preventing iatrogenic hypopituitarism or cerebrospinal fluid (CSF) leak. CPCs have previously been described as either small (<1.5-mm diameter) or large, with or without mass effect, but more recently have been classified into three types that more accurately describe size and associated pathologic appearances. Type 1, small incidental canals, may be found in patients with other craniofacial or neural congenital anomalies. Type 2 CPCs are medium-sized canals with ectopic pituitary tissue. Type 3 CPCs are large canals containing cephalocele (type 3A), tumors (type 3B), or both (type 3C). Type 3 large CPCs containing tumors and/or cephaloceles often contain ectopic pituitary tissue within the nasopharynx; thus, care should be taken during surgery to avoid iatrogenic hypopituitarism and/or CSF leak.

Transsphenoidal Encephalocele.
Transsphenoidal encephalocele ( Fig. 22-6 ) is thought to be due to the persistence of a craniopharyngeal canal. The clinical features include respiratory difficulties, feeding difficulties, episodes of recurrent meningitis, and endocrine abnormalities. Respiratory and feeding difficulties are related to a mass in the oral or nasal cavity. Associated congenital anomalies are seen in about a third of the patients and include hypertelorism, median nasal fissure, broad nasal root, and cleft lip or palate. The optic malformations include anophthalmia or microphthalmia, colobomas, retinal abnormalities, morning glory syndrome, and optic nerve or chiasm hypoplasia. The cerebral malformations include agenesis of the corpus callosum in up to 50% of cases, hydrocephalus, and pituitary dystopia or hypoplasia.

Imaging is necessary to confirm the diagnosis as well as to define any neural or vascular elements in the sac. Noncontrast CT with three-dimensional (3D) reconstruction is invaluable in reconstruction of the skull base defect. MRI is helpful to define the contents of the sac. Endocrine assessment is critical in every patient; hypothalamic-pituitary dysfunction is often present, with deficiency of antidiuretic hormone and growth hormones being the most common finding. Various surgical approaches have been described to repair these lesions, including transcranial, transbasal, and transpalatal approaches as well as endoscopic techniques.
Teratomas.
Teratomas are true neoplasms originating from pluripotent cells and are composed of tissues from all three germ layers. Teratomas occur in 1 of 4000 live births and show a female predominance, with head and neck teratomas accounting for less than 5% of the total. Head and neck teratomas are most commonly cervical, with the nasopharynx being the second commonest location. It is theorized that the teratoma interferes with fusion of embryonic tissues in the early developmental period and may have associated cranial anomalies like palatal fissures, hemicrania, and anencephaly. It may be sessile or pedunculated and often protrudes from the mouth. The most common presenting symptoms are upper airway obstruction, dysphagia, and failure to gain weight.
Accurate prenatal diagnosis is definitive to plan proper peripartum management. On prenatal ultrasound, polyhydramnios is often associated due to impaired swallowing. Maternal serum α-fetoprotein levels may be elevated. On CT and MRI, a teratoma can be suspected when a multiloculated lesion with focal areas of low (fat) attenuation and high signal intensity is seen on CT ( Fig. 22-7 ) and T1-weighted MRI, respectively. Areas of bone and tooth formation, if present, are characteristic, and these are in the form of mature tissue, unlike other bone-forming tumors. The primary differential diagnostic considerations include an encephalocele or meningoencephalocele (all noncalcified and mainly cystic) and hemangioma (mainly solid with phleboliths).

Inflammatory Lesions
Adenoidal Hypertrophy.
Adenoid is the condensation of lymphoid tissue on the posterosuperior wall of nasopharynx and part of Waldeyer’s ring (palatine tonsils, adenoids/nasopharyngeal tonsils, and lingual tonsils). Hypertrophy of these lymphoid tissues represents immunologic activity and is not a disease process per se. Adenoidal hypertrophy occurs physiologically in children between the ages of 6 and 10 years and atrophies by age 16. Males are more commonly involved (70%) than females, with the most commonly involved age group being 16 to 25 years (60%). Failure to visualize adenoidal tissue in a young child should raise the possibility of an immune deficiency state. The common causes of adenoidal hypertrophy in adults are chronic infection and allergy. Pollution and smoking are also important predisposing factors. Sometimes it is also associated with sinonasal malignancy, lymphoma, and human immunodeficiency virus (HIV) infection. Therefore all cases of adult adenoidal hypertrophy should be carefully evaluated to exclude underlying ominous causes.
Enlarged adenoids can become nearly the size of Ping-Pong balls and completely block airflow through the nasal passages. Infants present with difficulty in feeding, whereas older children present with symptoms of nasal obstruction, such as mouth breathing and snoring. Otitis media may result from encroachment on the orifice of the eustachian tube. Hypertrophic adenoids appear as a homogeneous fullness of the nasopharyngeal mucosal space, which obliterates the fossae of Rosenmüller and encroaches on the nasopharyngeal airway.
On CT scans, the adenoid is isodense to the underlying prevertebral muscles and may contain small cysts and calcifications ( Fig. 22-8 ). On T1-weighted MRI scans, it is isointense to the muscles as well but can be identified by its bright T2-weighted signal. On postcontrast scans, the pharyngobasilar fascia normally enhances as a thin continuous line outlining the deep adenoid surface. Identification of this line is a reliable sign of the noninvasive nature of the mass, although it does not guarantee benignity. In fact it may be impossible to differentiate between hypertrophic adenoids and nasopharyngeal lymphoma on imaging alone, and a biopsy is necessary for making a definitive diagnosis. Although adenoid hypertrophy is a benign lesion, it shows low apparent diffusion coefficient (ADC) values in both children and adults. This should be kept in mind to avoid possible misinterpretations as a malignant lesion. Adenoidectomy is indicated when hypertrophy results in nasopharyngeal airway obstruction, chronic otitis media, or serous middle ear effusion.

Pharyngitis.
Pharyngitis is defined as an infection or irritation of the pharynx. The etiology is usually infectious, with most cases being of viral origin and most bacterial cases attributable to group A streptococci (GAS). Other causes include allergy, trauma, toxins, and neoplasia. Imaging is usually not performed in acute and uncomplicated cases. Contrast-enhanced CT may demonstrate mild prominence of the posterior pharyngeal soft tissues, with linear striated enhancement.
Mucous Retention Cysts.
Postpharyngitis sequelae include formation of mucous retention cysts in the inflamed obstructed mucous glands. These retention cysts are usually asymptomatic but may be large enough to cause a superficial pharyngeal mass or otitis media secondary to eustachian tube orifice compromise. The cyst appears as a well-defined mass usually a few centimeters in diameter but can grow to a large size. Both CT and MRI demonstrate the appearance of a typical cyst (i.e., hypodense on CT scans, dark on T1-weighted images, and bright on T2-weighted images). However, high protein content increases CT density and T1-weighted signal intensity.
Retropharyngeal Abscess/Adenitis.
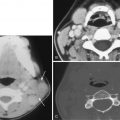
The most common source of retropharyngeal abscess in children is suppurative adenitis, which is difficult to differentiate from frank abscess. A retropharyngeal abscess commonly results from rupture of a suppurated retropharyngeal node. On imaging, abscesses appear as low-attenuation rim-enhancing collections with mass effect and flattening of the prevertebral muscles ( Fig. 22-9 ). They tend to displace the carotid sheath laterally and the parapharyngeal space anterolaterally. Life-threatening complications such as airway obstruction and aspiration may occur following rupture of an abscess into the airway. Treatment frequently requires surgical intervention for abscess drainage and intravenous antibiotics. Aggressive airway management is necessary in these patients to avoid airway obstruction and aspiration of pus.

MRI may help delineate retropharyngeal abscess better and differentiate it from cellulitis. Symmetric and smooth expansion of the retropharyngeal space by fluid density without an enhancing rim (termed retropharyngeal edema or nonabscess fluid ) may be seen in early infection, internal jugular vein thrombus, postradiation states, and prevertebral calcific tendonitis.
The differentiation of retropharyngeal edema from abscess on imaging is important because the former does not require surgical drainage. Retropharyngeal edema lacks an enhancing rim and is almost always confined to the level of oropharynx. Retropharyngeal abscesses may extend posteriorly to involve the danger and prevertebral spaces as well as cause osteomyelitis of the spine.
Benign Neoplasms
The commonest benign neoplasm of the nasopharynx is juvenile nasopharyngeal angiofibroma (JNA). Less frequently the nasopharynx can be a location for entities such as benign mixed tumors of salivary glands and posttransplant lymphoproliferative disorder.
Juvenile Nasopharyngeal Angiofibroma
Etiopathogenesis and symptoms.
JNA is a highly vascular benign but locally aggressive tumor arising from mesenchymal tissue and is typically seen in adolescent males, with a mean age of 15 years. The most frequent presenting symptoms are epistaxis and nasal speech and less commonly facial deformity.
Imaging features and spread patterns.
The exact site of origin is contentious because these masses usually present when they have reached considerable size. However, most authors agree that it originates from the posterior choanal tissues in the region of the sphenopalatine foramen ( Fig. 22-10A ). Anteriorly the tumor can extend into the nasal cavity and posteriorly through the choana into the contralateral nasal cavity. The ipsilateral nasal cavity may be expanded, with displacement of the nasal septum without frank erosion obliterating the contralateral nasal cavity (see Fig. 22-10B ). Superiorly it can extend into the ethmoid air cells and sphenoid sinus.

Posteriorly JNA extends to the nasopharynx, with potential airway compromise. The lesion can also erode the floor of sphenoid sinus into the sinus cavity. Most commonly, JNA spreads laterally through the pterygopalatine fossa, causing widening of this fossa with typical bowing of the posterior wall of maxillary antrum without osseous erosion. Focal erosion of the pterygoid lamina has been reported and is considered a more characteristic feature (see Fig. 22-10C ). Further lateral extension can occur into the pterygomaxillary fissure, reaching the infratemporal fossa inferiorly and temporal fossa superiorly. Extension into the orbit may occur from the pterygopalatine fossa through a widened inferior orbital fissure (see Fig. 22-10D ).
Superiorly, intracranial extension occurs through two pathways: either from the orbit through the superior orbital fissure into the middle cranial fossa or by directly eroding the roof of the sphenoid sinus into the central skull base into the cavernous sinus. Inferiorly the tumor extends into the buccal space laterally and the oropharynx and central oral cavity medially.
Staging.
Based on extent of spread on imaging, a grading system has been proposed (1996); grade 1 tumors are confined to the nasopharynx, grade 2 tumors extend into pterygopalatine fossa and masticator space/infratemporal fossa, and grade 3 tumors exhibit orbital or intracranial extension. The grading system can predict prognosis and possibility of recurrence and help plan the surgical approach. Preoperative embolization is usually performed prior to surgery. Recently a new staging has been proposed for JNA (University of Pittsburgh Medical Center, 2010), which relies not only on tumor size and extent but also on the route of intracranial extension and residual vascularity after angioembolization. The authors report improved prediction of intraoperative blood loss, the need for staged resections, and tumor recurrence.
Minor Salivary Gland Tumor/Benign Mixed Tumor.
Pleomorphic adenomas or benign mixed tumors most commonly arise from the soft palate, followed by tongue base or submucosa of the pharyngeal wall. Imaging features are nonspecific, and biopsy is required for diagnosis. The lesion is seen as a pedunculated homogenous mass with bright signal on T2-weighted sequences ( Fig. 22-11 ).

Posttransplant Lymphoproliferative Disorder.
Posttransplant lymphoproliferative disorder (PTLD) is a serious complication of organ transplantation. Although the precise etiology is unknown, the Epstein-Barr virus and immunosuppressive agents appear to be risk factors. The first manifestations of PTLD are frequently observed in the head and neck area, with adenoidal and/or tonsillar enlargement ( Fig. 22-12 ). PTLD remains an elusive disease, with diversity in its clinical presentation, course, and histology all contributing to the difficulty in diagnosing and treating these patients. The incidence after bone marrow or organ transplantation has been reported from 1% to 10% but varies based on the type of transplantation. Adenotonsillar PTLD is also more prevalent in younger patients, females, and liver and lung transplant recipients. A lower rate is reported in other immunocompromised patients, such as renal transplant and bone marrow recipients. Many of these cases are diagnosed when the child undergoes excision for adenotonsillar hypertrophy. It has been found that Waldeyer’s ring involvement appears to occur in both adults and children. Treatment continues to be controversial but is generally designed to accommodate the specific patient. Typically, although aggressive non-Hodgkin’s B-cell lymphoma is treated with chemotherapy, PTLD often can be treated with a major reduction of immunosuppressive medications. Surgical resection generally is attempted for limited disease.

Malignant Neoplasms
The commonest malignant tumor of the nasopharynx is nasopharyngeal carcinoma, which is a type of squamous cell carcinoma that comprises 70% of all neoplasms, followed by lymphomas that account for 20%. Minor salivary gland tumors, adenocarcinomas, rhabdomyosarcomas, extramedullary plasmacytoma, and melanomas are other less common neoplasms.
Nasopharyngeal Carcinoma
Etiopathogenesis and symptoms.
The highest incidence of nasopharyngeal carcinoma (NPC) is in southwest China, followed by Singapore and Hong Kong; Eskimoan and Mediterranean populations are also disproportionately affected. NPC has a seven times higher incidence in Americans of Chinese ancestry than in non–Chinese Americans. A higher incidence is also found in African Americans. The etiology appears to be the result of genetic susceptibility combined with environmental factors, some of which include consumption of nitrosamines in dry salted fish and chronic nasal infections with Epstein-Barr virus. The male-to-female incidence is 3 : 1, the peak age being 50 to 60 years, with a smaller peak in late childhood. NPC frequently presents with cervical metastatic nodes (in >70%) despite early small primary lesions, the other symptoms being otitis media (due to eustachian tube obstruction), sore throat, epistaxis, headache, and cranial nerve neuropathies in advanced cases.
Staging and treatment principles.
The criteria given in the seventh edition of the American Joint Committee on Cancer (AJCC) Cancer Staging Manual for tumor-node-metastasis (TNM) staging of NPC are provided in Box 22-1 . Surgery is unable to achieve clear resection margins, and radiotherapy (RT) remains the mainstay of treatment. Early-stage disease (T1 tumors without nodal metastases) treated with RT have a 5-year survival of 75% to 90%. In more advanced NPC, concurrent chemoradiation is the standard of care. Recently, transoral robotic surgery (TORS) has been used for local recurrence of NPC.
Primary Tumor (T)
TX Primary tumor cannot be assessed
T0 No evidence of primary tumor
Tis Carcinoma in situ
Nasopharynx
T1 Tumor confined to the nasopharynx, or tumor extends to oropharynx and/or nasal cavity without parapharyngeal extension *
* Note: Parapharyngeal extension denotes posterolateral extension of tumor.
T2 Tumor with parapharyngeal extension *
T3 Tumor involves bony structures of skull base and/or paranasal sinuses
T4 Tumor with intracranial extension and/or involvement of cranial nerves, hypopharynx, orbit or with extension to infratemporal fossa/masticator space
Oropharynx
T1 Tumor 2 cm or less in greatest dimension
T2 Tumor more than 2 cm but not more than 4 cm in greatest dimension
T3 Tumor more than 4 cm in greatest dimension or extension to lingual surface of epiglottis
T4a Moderately advanced local disease
Tumor invades the larynx, extrinsic muscle of tongue, medial pterygoid, hard palate, or mandible ^
^ Note: Mucosal extension to lingual surface of epiglottis from primary tumors of base tongue or vallecula does not constitute invasion of larynx.
T4b Very advanced local disease
Tumor invades lateral pterygoid muscle, pterygoid plates, lateral nasopharynx, or skull base or encases carotid artery
Regional Lymph Nodes (N)
Nasopharynx
The distribution and the prognostic impact of regional lymph node spread from nasopharyngeal cancer, particularly of the undifferentiated type, are different from those of other head and neck cancers and justify the use of a different N classification scheme.
NX —Regional lymph nodes cannot be assessed
N0 —No regional lymph node metastasis
N1 —Unilateral metastasis in lymph node(s), 6 cm or less in greatest dimension, above the supraclavicular fossa, and/or unilateral or bilateral, retropharyngeal lymph nodes, 6 cm or less, in greatest dimension. +
+ Midline nodes are considered ipsilateral nodes.
N2 —Bilateral metastasis in cervical lymph node(s), 6 cm or less in greatest dimension, above the supraclavicular fossa +
N3 —Metastasis in a lymph node(s) + > 6 cm and/or to supraclavicular fossa +
N3a —Greater than 6 cm in dimension
N3b —Extension to the supraclavicular fossa ++
++ Supraclavicular zone or fossa is relevant to the staging of nasopharyngeal carcinoma and is the triangular region originally described by Ho. It is defined by three points: (1) the superior margin of the sternal end of the clavicle, (2) the superior margin of the lateral end of the clavicle, (3) the point where the neck meets the shoulder. Note that this would include caudal portions of levels IV and VB. All cases with lymph nodes (whole or part) in the fossa are considered N3b.
Oropharynx ^^
^^ Note: Metastases at level VII are considered regional lymph node metastases.
NX —Regional lymph nodes cannot be assessed
N0 —No regional lymph node metastasis
N1 —Metastasis in a single ipsilateral lymph node, 3 cm or less in greatest dimension
N2 —Metastasis in a single ipsilateral lymph node, more than 3 cm but not more than 6 cm in greatest dimension, or in multiple ipsilateral lymph nodes, none more than 6 cm in greatest dimension, or in bilateral or contralateral lymph nodes, none more than 6 cm in greatest dimension
N2a —Metastasis in a single ipsilateral lymph node, more than 3 cm but not more than 6 cm in greatest dimension
N2b —Metastasis in multiple ipsilateral lymph nodes, none more than 6 cm in greatest dimension
N2c —Metastasis in bilateral or contralateral lymph nodes, none more than 6 cm in greatest dimension
N3 —Metastasis in a lymph node more than 6 cm in greatest dimension
Spread patterns.
NPC usually originates in the lateral pharyngeal recess ( Fig. 22-13A ) and spreads mucosally, submucosally, and beyond the confines of the nasopharynx as well. Spread to the torus tubarius results in obstruction of the eustachian tube with resultant serous otitis media. Spread to the nasal cavity anteriorly or inferiorly into oropharynx is classified as T1 disease. More commonly, early disease spread is seen posterolaterally into the parapharyngeal space (T2 disease) through the sinus of Morgagni.

Further posterolateral spread into the carotid space may occur, with invasion of CNs IX, X, XI, and XII, with resultant palsies (T4 disease). On axial images, hypoglossal nerve palsy results in fatty replacement of the affected side of the tongue along with prolapse of the hemitongue into the oropharynx, indicating hypotonia. Posterior spread to the retropharyngeal space, prevertebral muscles, and vertebrae may occur and is associated with increased incidence of cervical nodal metastases.
Superior extension into the sphenoid sinus and skull base upstages to T3 disease (see Fig. 22-13B ). Cranial spread can also occur directly through the foramen ovale (see Fig. 22-13C ) or foramen lacerum (located immediately superior to the lateral pharyngeal recess) into the middle cranial fossa or through the jugular foramen into the posterior cranial fossa, upstaging the tumor to T4 disease. Perineural spread along the mandibular nerve through the foramen ovale (see Fig. 22-13D ) can cause denervation atrophy of the muscles of mastication. Intracranial spread may extend to involve the dura mater, cavernous sinus, Meckel’s cave (see Fig. 22-13D ) and prepontine cistern. Lateral extension to the masticator space or infratemporal fossa is also T4 disease (see Fig. 22-13D ) and can cause trismus.
Anteriorly, lateral spread through the sphenopalatine foramen extends into the pterygopalatine fossa, from where tumor can spread (a) along the maxillary nerve ( Fig. 22-14A ) through the foramen rotundum to the intracranial cavity, (b) through the inferior orbital fissure into the orbital apex (T4 disease), (c) through the vidian canal into the carotid canal, (d) through the superior orbital fissure into the cranial cavity, and (e) through the pterygomaxillary fissure into the infratemporal fossa.

The primary echelon nodes in NPCs are level IIb and lateral retropharyngeal group (see Fig. 22-14B ), the other nodal levels being III, IV, and V. Spread to supraclavicular nodes is regarded as N3b (see Box 22-1 ).
Pretreatment imaging features.
On CT and MRI, NPC shows nonspecific imaging features and cannot be differentiated with certainty from lymphoma or minor salivary gland tumors. Hence the primary role of pretreatment imaging is complete disease staging to help plan optimal treatment. CT and MRI are complementary in evaluating the locoregional extent of NPC, but MRI is the technique of choice because it is more sensitive for depicting skull base invasion, perineural spread, invasion of dura and cavernous sinuses, and retropharyngeal adenopathy. Cortical erosion is best studied on CT.
Asymmetry of the nasopharynx is an early feature, but this may also be seen in asymmetric lymphoid hyperplasia; obliteration of the fat stripe between the tensor and levator veli palatini is a more reliable early imaging feature of NPC. The above features should prompt attention to serous mastoiditis, which suggests eustachian tube dysfunction, and lymphadenopathy in the ipsilateral neck, particularly in the retropharyngeal group.
In more advanced cases, imaging reveals invasion of adjacent spaces. Noncontrast and non–fat-suppressed T1-weighted sequences are most useful to demonstrate obliteration of the fat intensity between the adjacent space and tumor (which is isointense). NPC displays intermediate signal on T2-weighted sequences, with intense enhancement on postgadolinium images. Fat-suppressed contrast-enhanced images, particularly in the coronal plane, are valuable for confirming skull base marrow invasion (see Fig. 22-13B ), and for demonstrating orbital extension, perineural spread (see Figs. 22-13D and 14A ), and dural invasion. Thickening and enhancement of dura mater alone, however, could either represent intracranial extension of tumor or reactive inflammation (see Fig. 22-13C ).
Foraminal invasion due to perineural spread is seen as widening and increased enhancement (see Fig. 22-13D ). Denervation atrophy due to perineural spread is seen as hyperintense signal in the masticator muscles on T2-weighted images, with increased enhancement in the acute-subacute stage, and as diffuse muscle hyperintensity on both T1- and T2-weighted images, with reduced bulk in the chronic phase. Direct tumor invasion increases the muscle bulk, is focal rather than diffuse, and has signal intensity similar to tumor.
NPC and nodal metastases are usually FDG avid. Currently FDG-PET/CT is considered useful for mapping precise nodal burden, for evaluating distant metastases, and in the workup of unknown primary cancers presenting with cervical adenopathy. National Comprehensive Cancer Network (NCCN) practice guidelines recommend PET-CT to evaluate for distant metastases when stage III or IV disease is present. Imaging is also valuable for treatment planning. Accurate delineation of target volume with MRI as well as PET/CT results in more precise delivery of RT and indirectly improves local control and disease-specific survival. Imaging can also provide prognostic indicators. The most important prognostic criterion predictive of outcome and survival in NPC was CT-derived primary tumor volume greater than 50 mL during RT planning.
Response assessment and surveillance.
The NCCN guidelines recommend a baseline scan to be obtained within 6 months of completion of chemoradiation in patients with T3-4 and/or N2-3 disease. Further imaging is only recommended “as indicated based on signs/symptoms.”
Response assessment can be performed either with MRI or PET/CT, with equivalent reported accuracy for detection and restaging. CT has inferior soft tissue resolution to differentiate between posttreatment changes and residual or recurrent disease. A baseline posttreatment MRI scan is usually performed at 2 to 3 months after therapy because it is perceived to be valuable for detecting subclinical residual adenopathy and also serves as a baseline to detect future, more subtle recurrences. If the pretreatment study showed infiltration of the parapharyngeal space, pterygopalatine fossa, orbits, or the skull base, abnormal signal intensity may persist. The initial scan may also show significant posttreatment edema of the pharyngeal mucosa. There should be no residual enlarged lymph nodes, however; if these are found on the new baseline scan, a neck dissection is typically performed. The timing of PET/CT is critical, with a systematic review and meta-analysis reporting a high negative predictive value of 95% in scans done after 12 or more weeks. Earlier response assessment to chemoradiotherapy at 20 days and 50 days has been evaluated with diffusion-weighted MRI with promising results. High pretreatment ADC values and significant increase in ADC following initiation of induction chemotherapy showed better response to definitive treatment with concurrent chemoradiation.
Use of plasma Epstein-Barr virus (EBV) DNA for initial assessment, followed by PET/CT or MRI in positive cases, has been recommended. Wang et al. found plasma EBV DNA to have 100% accuracy in detecting recurrence of NPC. A recent study also found that patients with a negative 3-month PET/CT had limited benefit from subsequent PET surveillance.
On MRI, nonenhancing tissue with dark signal on T2-weignted sequences represents mature fibrosis. However, immature fibrosis (being cellular) may show intermediate to high T2-weighted signal intensity and may enhance, mimicking residual/recurrent disease. Following RT the nasopharynx may appear asymmetric and tends to lose its normal contours and develops a fibrosed appearance with effacement of the lateral pharyngeal recess primary site ( Fig. 22-15A-B ). Recurrent/residual tumor has bulging contours with enhancement (see Fig. 22-15C ) that can be detected with certainty on PET/CT as an FDG-avid lesion (see Fig. 22-15D ). Regression on serial scans may indicate post-RT changes, but unchanged lesions remain suspicious for occult or recurrent disease. Progressing masses or the appearance of a new node suggest recurrence. Another uncommon manifestation of recurrence is meningeal infiltration in the posterior fossa through the jugular foramen or foramen magnum without any visible nasopharyngeal component, which should prompt meticulous scrutiny of these regions.