Chapter 7 Physics of radiography
 In order to standardise the units of measurement used in science, the International System of Units (SI) was developed, which is derived from seven standard base units.
In order to standardise the units of measurement used in science, the International System of Units (SI) was developed, which is derived from seven standard base units. Some of these units (e.g. length (metre), mass (kilogram), electric current (ampere)) represent the fundamental measurements underpinning the physics of radiography.
Some of these units (e.g. length (metre), mass (kilogram), electric current (ampere)) represent the fundamental measurements underpinning the physics of radiography. From the standard base units, other SI units may be derived which are more applicable to radiography.
From the standard base units, other SI units may be derived which are more applicable to radiography. Electromagnetic radiation is a spectrum of energy levels containing a wide range of radiation types.
Electromagnetic radiation is a spectrum of energy levels containing a wide range of radiation types. The inverse square law determines the intensity of radiation reaching both the patient and the image receptor.
The inverse square law determines the intensity of radiation reaching both the patient and the image receptor.INTRODUCTION
It is essential that any practitioner operating within the realms of an imaging department and using ionising radiation has a sound knowledge base. In order to comprehend the various factors affecting the production of diagnostic images, there is a requirement to demonstrate an awareness of the fundamental definitions of classical physics and how these terms may be applied to radiography. An understanding of atomic structure, the electromagnetic spectrum, electricity, magnetism and the inverse square law are also essential principles that can be applied to radiography.
DERIVED SI UNITS RELEVANT TO RADIOGRAPHIC PRACTICE
Derived SI units result from a combination of the base units and some of them are used frequently in radiography. Practitioners are required to name them and define their values (Table 7.1). Some of these derived SI units are outlined below.
Table 7.1 Common SI units used in radiographic practice
| Term and SI unit | Definition | Application to radiography |
|---|---|---|
| Energy (joule; J) | The ability to do work | Production of X-rays |
| Mass (kilogram; kg) | A measure of the number of atoms and molecules in a body | Important when determining the radiation dose to a patient |
| Gray (joules per kilogram; Gy) | The energy imparted to a body by ionising radiation | Unit of absorbed radiation dose measurement |
| Sievert (joules per kilogram, Sv) | The energy imparted to a body by ionising radiation multiplied by the quality factor | Unit of radiation dose equivalent, which takes biological factors into account |
| Power (joules per second) | The rate of doing work | Output of X-ray generator |
| Electric current (ampere; A) | The movement of electrons flowing per unit time | Quantity of electrons flowing per unit time |
| Electric charge (coulomb; C) | 1 ampere flowing per second | Quantity of electrons flowing per second |
| Electrical potential (volt; V) | The force which moves electrons within a conductive material | Potential difference across an X-ray tube, acceleration of electrons and quality of X-ray beam |
| Frequency (hertz; Hz) | The number of cycles per second | Electromagnetic radiation |
ENERGY
This may be a difficult concept to understand but fundamentally energy is the ability to do work. This may be demonstrated in radiography as potentialenergy (PE), which is applied to the negative (cathode) and positive (anode) ends of an X-ray tube, subsequently causing the flow of electrons across the vacuum environment. This is kinetic energy (KE) and is subsequently converted into X-ray energy (photons) when the electrons interact with the anode material (tungsten atoms).
ATOMIC STRUCTURE
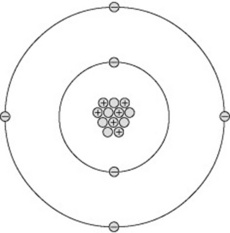
All matter consists of atoms and can be thought of as having a central nucleus surrounded by a cloud of particles called electrons (Fig. 7.1). The diameter of the nucleus is approximately 10−15 m.
The nucleus contains a number of particles called protons and neutrons, together termed nucleons. Each nucleon is nearly 2000 times the mass of an electron. This means the mass of an atom is concentrated in its nucleus, around which the much lighter electrons orbit. If a nucleus were scaled up to the centre spot of a football pitch, the electrons would start orbiting around the perimeter of the pitch in their various orbits stretching out for several miles. This analogy demonstrates why X-rays may pass through a body of material unattenuated, as the X-ray photons may simply pass ‘between’ the electron orbits and totally miss the nucleus of an atom.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree