Pineal Tumors
3.7.1 Pineoblastoma
Pineoblastomas belong to the group of PNETs, placing them in the same category as medulloblastomas and ependymoblastomas. Pineoblastomas occur mainly in very young patients. They are aggressive malignancies with a high mitotic rate. Histopathology shows areas of necrosis and intratumoral hemorrhage. Melanin-producing cells (high T1w signal) are occasionally detected.
MRI findings Pineoblastomas are hypo- to isointense on T1w images and hyperintense on T2w images. The tumor margins are irregular or lobulated. Contrast enhancement is usually only moderate and heterogeneous, which distinguishes pineoblastoma from pineocytoma. The tumors tend to infiltrate surrounding tissues, especially the corpus callosum, thalamus, and mesencephalon. Intratumoral hemorrhage and calcifications correlate with low signal intensity on T2*w imaging and SWI.
3.7.2 Pineocytoma
Pineocytoma is the benign variant of primary pineal tumors.
Epidemiology This tumor is very rare, comprising less than 1% of all primary brain tumors. Pineocytomas occur in the third and fourth decades of life, which is much later than pineoblastomas.
Clinical manifestations The tumors grow very slowly and may present clinically with slowly progressive hydrocephalus due to obstruction of the aqueduct.
Pathology Pineocytomas appear grossly as well-circumscribed, homogeneous tumors. The tumor matrix contains degenerative changes such as small hemorrhagic areas, cysts, or calcifications. Pineocytomas are classified as WHO grade II tumors.
MRI findings Consistent with their histopathology, pineocytomas appear on MRI as rounded tumors that are hypo- to isointense on T1w images and hyperintense on T2w images. They typically show intense, homogeneous enhancement.
Differential diagnosis Pineocytomas closely resemble ▶ germinomas on MRI, and the two entities may be indistinguishable based on MR appearance. Differentiation is aided by the determination of alpha-fetoprotein (AFP) and beta-human chorionic gonadotropin (β–hCG) levels in the CSF and serum. Rare cases of predominantly cystic pineocytomas have been reported, and these lesions are very difficult to distinguish from a simple pineal cyst ( ▶ Fig. 3.38).

Fig. 3.38 Cystic pineocytoma. (a) Sagittal T2w image. Unlike a pure pineal cyst, this small tumor exhibits soft-tissue mural nodules in addition to a cystic component. (b) Sagittal T1w image after contrast administration shows enhancement of the soft-tissue components (arrows).
3.7.3 Pineal Cyst
Epidemiology and clinical manifestations Pineal cyst is a common finding that is detected in up to 40% of all autopsies and up to 10% of all MRI examinations. Generally these cysts are asymptomatic, but cyst enlargement may cause a local mass effect with compression of the upper quadrigeminal plate (Parinaud symptoms) or aqueduct (hydrocephalus).
Pathology The etiopathogenesis of pineal cysts is uncertain, and various theories have been proposed. Some authors believe that they result from a persistent diverticulum arising from the pineal recess of the third ventricle (Cooper hypothesis), while others attribute them to a degenerative process in the pineal gland. As a general rule, pineal cysts are described as such when they reach a size of at least 5 mm, but there are reports of pineal cysts less than 2 mm in diameter.
MRI findings The cyst contents are hypointense on T1w images and hyperintense on T2w images. They may be isointense or hyperintense on PDw and FLAIR images, depending on the protein content of the cyst fluid. Cyst wall enhancement is found in 50% of benign pineal cysts ( ▶ Fig. 3.39). Management is often difficult in the case of asymptomatic pineal cysts that are detected incidentally. Surgical treatment is recommended for large cysts causing significant compression of the quadrigeminal plate and/or obstruction of CSF flow with associated clinical symptoms. Smaller asymptomatic cysts are most commonly found, but MRI cannot distinguish them from cystic pineocytomas, which may undergo progression or even acute intralesional hemorrhage with mass effect (“pineal apoplexy”). In any case, a blanket recommendation for the follow-up of all incidentally detected pineal cysts is impractical. Additional imaging criteria or clinical arguments would be needed to justify the MRI follow-up of an otherwise asymptomatic pineal cyst. Benign pineal cysts are generally detected between puberty and 40 years of age. Patients with pineal cysts outside this age range would be potential candidates for follow-up. Marked thickening of the cyst wall (>2 mm) or the detection of irregularly enhancing solid components would also be more suggestive of a tumor than a benign cyst. Based on recommendations in the literature, all other pineal cysts that appear benign on MRI require only clinical follow-up, even when larger than 1 cm. A 1-cm cyst diameter was considered the cutoff value for follow-up in earlier studies.

Fig. 3.39 Pineal cyst. (a) Incidental finding of a unilocular, thin-walled cyst of the pineal body (arrows). The cyst contents have slightly different signal intensity than CSF in the sagittal CISS sequence (0.75 mm slice thickness). The cyst is not compressing the quadrigeminal plate. (b) Axial T1w image after contrast administration. A portion of the cyst wall enhances, but nodular wall thickenings are not present.
Differential diagnosis The differential diagnosis of pineal cysts should include a predominantly cystic tumor such as cystic pineocytoma or germinoma. It also includes inflammatory processes such as histiocytosis and sarcoidosis, which may have a purely cystic appearance on MRI.
3.7.4 Germinoma
Germ cells are disseminated throughout the body of the developing embryo. Germ cell tumors, on the other hand, are limited to the gonads, diencephalon (pineal and suprasellar regions), and mediastinum. Germ cell tumors of the diencephalon are subdivided into germinomas (approximately 50%), teratomas (20%), and mixed germ cell tumors (30%).
Epidemiology Three-fourths of patients are between 10 and 20 years of age at clinical presentation, and 95% of patients are under age 35. Males are predominantly affected.
Clinical manifestations and treatment Clinical symptoms are determined by tumor location: impaired ocular motility due to compression of the upper quadrigeminal plate, and impaired CSF flow with hydrocephalus due to obstruction of the aqueduct. Germ cell tumors may also cause precocious puberty due to destruction of the pituitary gland or hypothalamus. Elevated β-hCG and AFP levels are typically found in the CSF and serum (important differentiating criterion). Germinomas are highly radiosensitive, so radiation is a first-line treatment option in diagnosed cases. Biopsy may even be omitted in cases where typical radiologic and laboratory findings are sufficient to establish the diagnosis.
MRI findings The MRI appearance of germinomas is fairly nonspecific: The tumors are relatively isointense to gray matter in all sequences. Hyperintense components are occasionally found in T1w images and hypointense components in T2w images. Enhancement characteristics are also variable, with different studies reporting very homogeneous or very heterogeneous enhancement. Unlike their variable appearance on MRI, germinomas are markedly hyperdense on noncontrast CT, and this provides a useful differentiating criterion ( ▶ Fig. 3.40).

Fig. 3.40 Germinoma. (a) Axial CT shows a hyperdense tumor in the pineal region with central calcifications. Compression of the quadrigeminal plate has caused obstructive hydrocephalus with corresponding ventricular dilation and transependymal CSF flow. (b) Axial T2w image. The tumor has cauliflower-like margins and small intratumoral cysts with fluid levels. (c) Sagittal T1w image. The T1w hyperintensities suggest intratumoral hemorrhage. Note the pressure-induced depression in the floor of the third ventricle. (d) Sagittal T1w after contrast administration shows intense enhancement.
Tips and Tricks
If MRI is suspicious for germinoma, confirm the diagnosis by noncontrast CT.
If the tumor appears relatively dense or hyperdense, the next diagnostic step is to determine β–hCG and AFP levels in the serum and CSF. Differentiation from other tumors of the pineal region is very difficult based on MRI morphology alone.
3.7.5 Pineal Teratoma
Teratoma is the second most common pineal tumor (15%). Like germinomas, they show a male predilection and are particularly common in younger children. Teratomas may be benign or malignant. Benign teratomas are relatively well circumscribed and have a lobulated or partially cystic appearance. Typically they contain a number of diverse tissue types (ectoderm, mesoderm, and endoderm). Malignant transformation of an initially benign teratoma to teratocarcinoma or teratosarcoma may rarely occur. Imaging presentation is highly variable. Teratomas usually appear on MRI as heterogeneous lesions with calcifications and a mixture of cysts, fat, and connective tissue elements.
3.8 Tumors of the Sellar Region
3.8.1 Pituitary Adenoma
Pituitary adenomas originate from the secretory cells of the adenohypophysis (anterior lobe of the pituitary). These secretory cells synthesize six different large peptide hormones:
Growth hormone (GH).
Prolactin (PRL).
Adrenocorticotropic hormone (ACTH).
Thyroid-stimulating hormone (TSH).
Follicle-stimulating hormone (FSH).
Luteinizing hormone (LH).
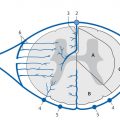
The neurohypophysis (posterior lobe of the pituitary) is connected to the hypothalamus by the infundibulum. Two hormones produced by the hypothalamus are stored and later released by the neurohypophysis: oxytocin and vasopressin. The cells that secrete PRL and GH are located mainly in the lateral portions of the adenohypophysis. The other hormones are produced in the central third of the adenohypophysis ( ▶ Fig. 3.41).

Fig. 3.41 Distribution of hormone-producing cells in the pituitary gland.
ACTH = adrenocorticotropic hormone
ADH = antidiuretic hormone
FSH = follicle-stimulating hormone
GH = growth hormone
OXY = oxytocin
PRL = prolactin
TSH = thyroid-stimulating hormone
Epidemiology Pituitary adenomas are the most common neoplasm of the sellar region and comprise approximately 10–15% of all intracranial neoplasms. Their incidence in the general population is 1 to 15:100,000. Most occur between the third and sixth decades of life, with a 2:1 female preponderance. Approximately 25% of adenomas produce prolactin (prolactinomas), and another 25% are “nonfunctioning” (endocrine-inactive). The next most common functioning (hormone-producing) adenomas, comprising just 10% of all adenomas, are GH- or ACTH-producing tumors
Clinical manifestations The clinical presentation is determined either by the endocrine effects of the adenoma or by the local mass effect from a macroadenoma.
Nonfunctioning adenomas: Many nonfunctioning adenomas (formerly called “chromophobic” adenomas) are not completely inactive but produce small amounts of various hormones. Pressure on the other portions of the adenohypophysis can lead to secondary endocrine dysfunction that may include secondary hyperprolactinemia. The latter effect is usually less pronounced than with a primary prolactinoma, however. Otherwise the nonfunctioning adenomas become symptomatic due to their mass effect on adjacent structures. Tumor extension in the cranial or frontal direction may elevate and compress the optic chiasm and prechiasmal optic nerve. More lateral extension into the cavernous sinus may compress the cranial nerves at that level (III, IV, and VI). Adenomas may even encase the internal carotid artery within the cavernous sinus.
Functioning adenomas: Most functioning adenomas are less than 10 mm in diameter when they produce clinical manifestations; they are called microadenomas. A particularly common presentation is amenorrhea in young women due to prolactinoma. A PRL level higher than 150 ng/mL is suggestive of primary prolactinemia.
Treatment Macroadenomas causing a local mass effect should be treated surgically to decompress or protect neuronal structures, especially the optic tract. On the other hand, lateral tumor components usually cannot be excised from the cavernous sinus. Macroadenomas may show infra-, intra-, or suprasellar extension or may grow laterally into the cavernous sinus. The surgical approach is tailored to the direction of tumor extension. Tumors growing predominantly in the midline can be treated through a transsphenoidal approach. Tumors with lateral or superolateral extension can be approached through a temporal craniotomy. In the transsphenoidal approach, the sphenoid sinus should be packed with fat, muscle, or dura at the end of the operation to prevent CSF leakage. Initial medical therapy (bromocriptine) should be tried for microadenomas that do not threaten the optic tract, especially small prolactinomas. Bromocriptine therapy may lead to acute intratumoral hemorrhage, however, with associated acute expansion of the adenoma.
Pathology Pituitary adenomas tend to be very slow-growing tumors. Tumors smaller than 10 mm in diameter are classified as microadenomas. Calcifications in adenomas are very unusual, but intratumoral hemorrhage may occur in prolactinomas treated with bromocriptine. In addition to solid and mixed solid-cystic tumors, adenomas may also be purely cystic or predominantly cystic. Acute bleeding into the pituitary gland or into a pituitary tumor (“acute pituitary apoplexy”) may cause a significant volume increase resulting in acute compression of the optic tract. Besides acute hormonal dysfunction, these patients complain of acute headaches and visual disturbances.
MRI findings In the diagnostic management of pituitary tumors, it is helpful to distinguish between microadenomas and macroadenomas because different questions must be addressed for these lesions, and they have different therapeutic implications.
Microadenomas The main imaging goals are to identify the microadenoma as such and determine its location. Some patients may have borderline hormone values that, while suspicious for a pituitary adenoma, do not establish its presence. Imaging is of key importance in these cases. High-resolution coronal T2w images (slice thickness <3 mm) may demonstrate microadenomas as either a hyperintense or hypointense focus in the pituitary tissue. Most microadenomas appear as a hypointense lesion on unenhanced T1w images. They are detectable, however, only if thin-slice imaging is used. Three-dimensional sequences can also add significant information, whereas dynamic T1w imaging has not proven very rewarding. Contrast-enhanced T1w imaging for microadenoma detection should employ only half the usual contrast dose to avoid “washout” of the pituitary tumor, which would mask the microadenoma ( ▶ Fig. 3.42). Indirect signs of mass effect are also helpful for detecting microadenomas: elevation of the sellar diaphragm, displacement of the infundibulum ( ▶ Fig. 3.43), and erosion of the sellar floor.

Fig. 3.42 Pituitary microadenoma. (a) Microadenoma in the right anterior lobe of the pituitary gland shows inhomogeneous signal in the T2w image with iso-, hyper- and hypointense components. The gland appears only slightly expanded on the right side. (b) The tumor is poorly visualized in the unenhanced T1w image. (c) After contrast administration (half-dose), the tumor appears as a well-defined hypoperfused mass that is hypointense to normal pituitary tissue.

Fig. 3.43 Pituitary microprolactinoma. (a) The small adenoma, located in the left basal area, is not detectable in the T2w image. (b) It is not visualized in the unenhanced T1w image. (c) The adenoma (arrows) is visible only after contrast administration. The infundibulum is displaced slightly toward the right side.
Note
Use half-dose MRI contrast for detecting pituitary microadenomas.
Macroadenomas The main imaging goals for these lesions are to define tumor extent, identify the optic tract and its various parts, detect possible extension into the cavernous sinus, and if necessary differentiate the macroadenoma from other intra- or parasellar tumor entities. Adenomas are usually isointense to gray matter in all sequences, but occasional tumors are purely or predominantly cystic. Solid tumor components generally show intense, homogeneous enhancement after contrast administration. MRA can document lateral displacement of the carotid arteries. Unlike meningiomas, adenomas generally do not narrow the internal carotid artery. With suprasellar extension, the tumor is often constricted at its passage through the sellar diaphragm, creating a “figure 8” appearance on coronal images ( ▶ Fig. 3.44, ▶ Fig. 3.45).

Fig. 3.44 Pituitary macroadenoma (nonfunctioning). The tumor appears as a hypointense mass (a, arrows) that shows homogeneous enhancement (b,c), elevates the optic chiasm (a), and extends to the right cavernous sinus, displacing the carotid artery laterally. The adenoma appears constricted where it passes through the sellar diaphragm. The tumor has not yet caused visual impairment.
(a) Coronal T1w image without contrast. (b) Coronal T1w image after contrast administration. (c) Sagittal T1w image after contrast administration.

Fig. 3.45 Pituitary macroadenoma (nonfunctioning). (a) T2w image shows a slightly hyperintense tumor compressing and displacing the optic chiasm, making it difficult to identify. (b) The carotid arteries are displaced laterally in their C1 segment. (c) The tumor has developed a cyst at its posterior border. The patient has significant visual impairment.
Tips and Tricks
On immediate postoperative MRI for exclusion of residual tumor after a transsphenoidal resection, enhancement may initially be absent in solid residual tumor. Apparently this is because the transsphenoidal resection reduces tumor vascularity to such a degree that the residual tumor does not enhance. But when imaging is repeated several weeks later, enhancement will again be present in the residual tumor tissue. Serial follow-up examinations are recommended in cases of this kind.
Differential diagnosis The main entities requiring differentiation from pituitary adenoma in adults are meningiomas of the tuberculum sellae or cavernous sinus. The main differential diagnosis in children is craniopharyngioma, in which the cyst contents often appear very hyperintense in various sequences due to their high cholesterol content. Smaller masses require differentiation from dysontogenetic tumors (Rathke cleft cyst, dermoid, epidermoid). Large, partially thrombosed carotid aneurysms with medial extension can mimic an intrasellar tumor. Metastases and abscesses have been reported in the anterior pituitary tissue or infundibulum. Acute hemorrhage into the pituitary, causing “pituitary apoplexy,” can be identified as blood in GRE or echo planar imaging (EPI) sequences. Germinomas, optic nerve gliomas, and hypothalamic gliomas are primary suprasellar tumors that can be distinguished by their original location.
3.8.2 Craniopharyngioma
Craniopharyngiomas are generally benign, potentially cystic epithelial tumors of the sellar region that are most commonly found in children and young adults. They arise from epithelial rests along the involuted pituitary Rathke duct.
Epidemiology Craniopharyngiomas account for 3 to 5% of primary intracranial tumors. Approximately 50% of craniopharyngiomas are observed in the first two decades of life. There is a similar incidence in males and females. Two main types of craniopharyngioma are distinguished: adamantinomatous and papillary. Papillary craniopharyngiomas are found exclusively in adults. Pure intrasellar craniopharyngiomas are rare; most are entirely suprasellar or have a predominantly suprasellar component plus a small intrasellar component. This characteristic location aids in differentiation from adenomas.
Clinical manifestations and treatment Headaches are the most common symptom, followed by endocrine deficits and visual impairment due to direct compression of the optic tract. The treatment of choice is surgical resection and cyst evacuation. A complete resection often cannot be attained; postoperative radiotherapy is reportedly useful for lowering the recurrence rate. Recurrent tumors can be treated by reoperation or interstitial irradiation. The overall recurrence rate is relatively high.
Pathology Two different histologic types of craniopharyngioma are distinguished:
Adamantinomatous type: This type is derived from the adamantine of the jaw and from odontogenic cysts.
Papillary variant: This type arises from a Rathke cleft cyst; thus it tends to occur in younger patients and has a higher recurrence rate.
Craniopharyngiomas consist of a mixture of cystic and solid components, and some may present entirely as a large cystic mass. Focal calcifications are found in the capsule and in solid components. The cysts typically contain a thick, oily fluid (“machine oil”). Calcifications and the oily cystic fluid are most characteristic of adamantinomatous craniopharyngiomas.
MRI findings The most common imaging appearance of craniopharyngioma is a cyst, which in some tumors shows markedly high signal intensity in various sequences. In atypical cases, however, the cystic fluid may be hypointense in T1w images and hyperintense only in T2w images ( ▶ Fig. 3.46, ▶ Fig. 3.47). The detection of hyperintense cystic fluid in T1w or PDw images is virtually pathognomonic for craniopharyngioma. The solid tumor component shows intense but heterogeneous enhancement. Larger tumors may give rise to cystlike “drop metastases” spreading as far as the posterior cranial fossa and around the brainstem. These metastases also exhibit very hyperintense cystic contents.

Fig. 3.46 Papillary craniopharyngioma. (a) The predominantly cystic tumor is hyperintense in the T2w image. (b) It is hypointense in the T1w image. (c) Contrast enhancement is confined to a small, lateral tumor component.

Fig. 3.47 Adamantinomatous craniopharyngioma. (a) The cholesterol-rich cyst contents are hyperintense in the T1w image. (b) The material has low signal intensity in the T2w image.
Differential diagnosis Although typical MRI findings have been described for adamantinomatous and papillary craniopharyngiomas, unfortunately the two entities cannot be distinguished from each other with absolute confidence. In rare cases even the papillary type may display hyperintense cystic components and calcifications, and conversely the adamantinomatous type may show papillary features. With tumors at a more lateral and posterior location, especially at the petrous apex, the differential diagnosis should include cholesterol granuloma, which also exhibits very hyperintense fluid in various sequences. Differentiation is also required from common tumors of the sellar and suprasellar region:
Pituitary adenoma.
Epidermoid and dermoid cyst.
Rathke cleft cyst.
Cystic glioma.
Germinoma.
Lymphoma.
Meningioma.
3.8.3 Dysontogenetic Lesions
The most common dysontogenetic lesions of the pituitary are nonneoplastic cysts:
Pars intermedia cyst.
Colloid cyst.
Rathke cleft cyst.
3.8.3.1 Pars Intermedia and Colloid Cysts
Pars intermedia cysts are located between the adenohypophysis and neurohypophysis and have arachnoid or epithelial components. The cyst contents have fluid signal intensity in the various MRI sequences. Pathologic enhancement does not occur. Generally the cysts are relatively small and may be mistaken for microadenomas ( ▶ Fig. 3.48).

Fig. 3.48 Pars intermedia cyst. The cyst (arrows) is located between the adenohypophysis and neurohypophysis and is isointense to CSF in the unenhanced image.
Colloid cysts also have an arachnoid or epithelial origin but occasionally appear hyperintense in various sequences due to their more protein-rich fluid content.
3.8.3.2 Rathke Cleft Cyst
Epidemiology Rathke cleft cyst is the most common type of cyst found at this location. Approximately 70% are intra- and suprasellar, and 25% are purely intrasellar. Small cleft cysts are found in up to 25% of autopsy series.
Pathology The Rathke cleft cyst arises from remnants of the embryonic craniopharyngeal duct. Occasionally these remnants give rise to a macroscopic cyst, which is then called a Rathke cleft cyst. It is typically located at an intra- or suprasellar site in the midline, i.e., on the infundibulum.
Clinical manifestations and treatment Intrasellar cysts are usually asymptomatic because they are too small to compress surrounding structures. Larger cysts may produce symptomatic mass effects, in which case the treatment of choice is partial excision of the cyst wall. The recurrence rate is low.
MRI and CT findings The cyst usually has general fluid signal intensity on MRI, and CT also demonstrates a hypodense mass. In some cases, however, CT may show hyperdense cysts whose contents are hypointense on T2w MRI and hyperintense on T1w images. Typically the cyst contents do not enhance, and this provides the only reliable differentiating criterion, although the cyst wall may enhance. The detection of an intracystic nodule has been described as a special MRI feature for identifying a Rathke cleft cyst with variable signal characteristics in different sequences. These cyst nodules are most clearly visualized in thin-slice T2w images and are found in up to 77% of all Rathke cleft cysts.
3.8.3.3 Epidermoid
Epidermoids may also occur in the suprasellar region. They have the same MRI appearance as epidermoids in other regions (see ▶ 3.11.4).
3.8.3.4 Dermoid
Intracranial dermoids are uncommon cystic lesions that occur predominantly in the posterior cranial fossa. When supratentorial, however, they have a predilection for the sellar and suprasellar region. They arise from embryonic ectodermal tissue and are typically located in the midline. These dermoids often contain very lipid-rich fluid that has high T1w signal intensity and may have high or low T2w signal intensity ( ▶ Fig. 3.49). On CT the lesions are also relatively well-defined with a rounded shape and fat-rich contents. Calcifications may be present in the peripheral capsule, and some degree of capsular enhancement may occur.

Fig. 3.49 Intrasellar dermoid cyst. (a) The dermoid appears hyperintense in the unenhanced T1w image. (b) The dermoid is masked after contrast administration. (c) The absence of enhancement is clearly demonstrated in the subtraction image. (d) The dermoid is hypointense in the T2w image.
Note
A feared complication is dermoid rupture with spillage of the lipid-rich cyst contents into the subarachnoid space. This may incite a chemical meningitis with a severe clinical course (vasospasm, infarction).
3.8.3.5 Ectopic Neurohypophysis
An ectopic posterior pituitary with dysplasia or even aplasia of the infundibulum is another important differential diagnosis for suprasellar lesions. The ectopic neurohypophysis appears on T1w images as a hyperintense “bright spot” at the level of the hypothalamus or between the hypothalamus and adenohypophysis, in the area where the posterior lobe normally descends into the pituitary fossa. The ectopic neurohypophysis is demonstrated particularly well on sagittal T1w images ( ▶ Fig. 3.50). Usually the sella is correspondingly small. The patient presents with pituitary dysfunction, often with pituitary dwarfism. Detection of the hypothalamic bright spot should direct attention to the sella to check for absence of the neurohypophysis. Otherwise the differential diagnosis of a hyperintense hypothalamic lesion would include a Rathke cleft cyst, craniopharyngioma, dermoid, and hypothalamic hemorrhage.

Fig. 3.50 Ectopic neurohypophysis. The ectopic position of the hyperintense neurohypophysis (arrows) is best appreciated on sagittal (a) and coronal (b) T1w images without contrast. Note absence of the infundibulum.
(a) Unenhanced sagittal T1w image. (b) Unenhanced coronal T1w image.
3.8.4 Germinoma
The suprasellar region is the second most common location of germinomas (germ cell tumors). Germinomas in this region are located in the hypothalamic–neurohypophyseal axis and invariably involve the pituitary infundibulum. In rare cases the tumor may be confined entirely to the infundibulum.
Epidemiology and clinical manifestations Most patients are under 30 years of age and have endocrine disorders such as diabetes insipidus or panhypopituitarism. Rarely, precocious puberty may also occur. Due to the rather slow growth rate of germinomas, mass effects tend to develop gradually and, when occurring at critical sites, may cause obstruction of CSF flow. The dominant symptoms, however, are endocrine dysfunction and visual impairment (optic chiasm). As with germinomas of the pineal region, β–hCG and AFP levels are elevated in the serum and CSF.
MRI findings With smaller tumors, MRI shows marked expansion of the infundibulum. The hyperintense signal of the neurohypophysis is usually absent on sagittal T1w images (which explains the presence of diabetes insipidus). The tumor is usually isointense to cortex on T1w images and shows intense, homogeneous contrast enhancement. The tumors show variable signal characteristics on T2w images ( ▶ Fig. 3.51). Germinomas are hyperdense on CT, as in the pineal gland, with no evidence of calcifications.

Fig. 3.51 Suprasellar germinoma. (a) T1w image shows a hypointense tumor. The neurohypophysis is absent. (b) Postcontrast image shows homogeneous enhancement of the tumor and adenohypophysis. (c) The tumor is hypointense on the T2w image.
3.8.5 Chordoma and Chondroma
3.8.5.1 Chordoma
Pathology and clinical manifestations Chordomas are very slow-growing tumors. They originate from remnants of the primitive notochord and are usually located on the midline in the clival region. Most of these tumors have a very slow growth rate and, as a result, cause only mild clinical symptoms. Some chordomas may undergo malignant transformation, however, and these lesions are associated with rapid and extensive bone destruction. Large chordomas may destroy all of the anterior skull base. They may spread into the nasopharynx, penetrate the dura and enter the subarachnoid space, even invading the brain. Pathologically, the tumors have a gelatinous consistency and are subdivided into chordomas and chondroid chondromas. The latter type has a better overall prognosis.
Epidemiology The tumors are generally diagnosed in middle-aged adults but may occasionally occur in children. Males are more commonly affected than females.
MRI findings Chordomas are isointense or slightly hypointense to surrounding brain tissue on conventional T1w images and contrast well with the fat in the clivus. Small punctate hyperintensities within the tumor reflect bleeding into the tumor matrix. GRE images may show intratumoral calcifications as signal voids. The T2w findings are typical, with chordomas showing very high signal intensity due to their high intracellular fluid content (gelatinous). Most intracranial chordomas show moderate to pronounced enhancement after contrast administration. Enhancement may also be very slight or absent, however. An absence of enhancement correlates with the proportion of mucous material in the tumor. Enhancement may also show a somewhat honeycomb pattern in some cases ( ▶ Fig. 3.52). Fat-suppressed axial T1w images after contrast administration are helpful in defining the tumor boundaries. Fat-suppressed T2w images (short-tau inversion recovery [STIR]) are even better, however, for defining the full extent of the tumor in relation to surrounding tissue. MRA is helpful in documenting the integrity of the basal cerebral arteries and detecting involvement of the carotid and basilar arteries. Axial thin-section CT scans with a bone window setting are better for detecting bone destruction.

Fig. 3.52 Clivus chordoma. (a) In a T2w STIR image, the tumor shows typical high signal intensity with some small cystic components. The extent of the tumor is well defined relative to surrounding structures. (b) Unenhanced T1w image. (c) Postcontrast image shows honeycomb enhancement of the tumor matrix. The tumor has invaded the cranial cavity through the posterior border of the clivus and is compressing the medulla.
Treatment Today these tumors are effectively treated by modern skull base surgery. Given its proximity to critical anatomic structures (brainstem, cranial nerves), this tumor entity is also well suited for proton-beam therapy, which provides a very sharp dose fall-off at the tumor boundaries. Skull base surgery combined with radiotherapy appears to be the most effective treatment strategy for these tumors.
Tips and Tricks
The surgical treatment of chordomas should be followed by early postoperative imaging for baseline documentation so that tumor progression can be promptly recognized.
3.8.5.2 Chondroma
Pathology The skull base is also a site of predilection for intracranial chondromas. These tumors are presumed to originate from embryonic cartilage remnants, but there are chondromas that also arise from the dura. Unlike chordomas, chondromas tend to have a more lateral origin located away from the midline. Chondroma may undergo malignant transformation to chondrosarcoma.
MRI findings Chondromas and chordomas may resemble each other on MRI: Chondromas are slightly hypointense on T1w images and may be very hyperintense on T2w images. Often, however, they appear to have mixed hypo- to hyperintense signal on T2w images. They apparently show less enhancement than chordomas. As with other skull base lesions, MRA is an important arterial and venous study for evaluating the integrity of the basal cerebral vessels.
3.8.6 Optic Nerve Glioma
Epidemiology Gliomas of the optic nerve are much less common than gliomas of the brain.
Pathology Most prechiasmal optic nerve gliomas are very slow-growing, low-grade tumors (WHO grade I = pilocytic astrocytoma). Gliomas of the optic chiasm or optic tracts, on the other hand, have a more anaplastic appearance. Optic nerve gliomas are most commonly found in patients with neurofibromatosis type 1; up to 20% of these patients have unilateral or bilateral optic nerve gliomas.